The retrograde spread of synaptic potentials and recruitment of presynaptic inputs
- PMID: 15788765
- PMCID: PMC6725090
- DOI: 10.1523/JNEUROSCI.4433-04.2005
The retrograde spread of synaptic potentials and recruitment of presynaptic inputs
Abstract
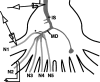
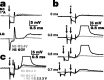
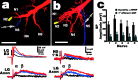
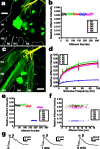
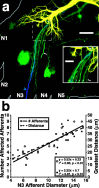
Lateral excitation is a mechanism for amplifying coordinated input to postsynaptic neurons that has been described recently in several species. Here, we describe how a postsynaptic neuron, the lateral giant (LG) escape command neuron, enhances lateral excitation among its presynaptic mechanosensory afferents in the crayfish tailfan. A lateral excitatory network exists among electrically coupled tailfan primary afferents, mediated through central electrical synapses. EPSPs elicited in LG dendrites as a result of mechanosensory stimulation spread antidromically back through electrical junctions to unstimulated afferents, summate with EPSPs elicited through direct afferent-to-afferent connections, and contribute to recruitment of these afferents. Antidromic potentials are larger if the afferent is closer to the initial input on LG dendrites, which could create a spatial filtering mechanism within the network. This pathway also broadens the temporal window over which lateral excitation can occur, because of the delay required for EPSPs to spread through the large LG dendrites. The delay allows subthreshold inputs to the LG to have a priming effect on the lateral excitatory network and lowers the threshold of the network in response to a second, short-latency stimulus. Retrograde communication within neuronal pathways has been described in a number of vertebrate and invertebrate species. A mechanism of antidromic passage of depolarizing current from a neuron to its presynaptic afferents, similar to that described here in an invertebrate, is also present in a vertebrate (fish). This raises the possibility that short-term retrograde modulation of presynaptic elements through electrical junctions may be common.
Figures

Similar articles
-
A lateral excitatory network in the escape circuit of crayfish.J Neurosci. 2002 Oct 15;22(20):9078-85. doi: 10.1523/JNEUROSCI.22-20-09078.2002. J Neurosci. 2002. PMID: 12388615 Free PMC article.
-
The onset of response habituation during the growth of the lateral giant neuron of crayfish.J Neurophysiol. 1994 Aug;72(2):890-8. doi: 10.1152/jn.1994.72.2.890. J Neurophysiol. 1994. PMID: 7983544
-
Changes in synaptic integration during the growth of the lateral giant neuron of crayfish.J Neurophysiol. 1994 Aug;72(2):899-908. doi: 10.1152/jn.1994.72.2.899. J Neurophysiol. 1994. PMID: 7983545
-
Local and afferent synaptic pathways in the striatal microcircuitry.Curr Opin Neurobiol. 2015 Aug;33:182-7. doi: 10.1016/j.conb.2015.05.002. Epub 2015 Jun 4. Curr Opin Neurobiol. 2015. PMID: 26051382 Review.
-
Functional interaction of electrical coupling and H-current and its putative impact on inhibitory transmission.Neuroscience. 2025 May 14;574:13-20. doi: 10.1016/j.neuroscience.2025.03.051. Epub 2025 Mar 25. Neuroscience. 2025. PMID: 40147622 Review.
Cited by
-
Species-specific behavioral patterns correlate with differences in synaptic connections between homologous mechanosensory neurons.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Mar;196(3):181-97. doi: 10.1007/s00359-010-0503-y. Epub 2010 Feb 5. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 20135128 Free PMC article.
-
D-type K+ current rules the function of electrically coupled neurons in a species-specific fashion.J Gen Physiol. 2023 Sep 4;155(9):e202313353. doi: 10.1085/jgp.202313353. Epub 2023 Jun 28. J Gen Physiol. 2023. PMID: 37378665 Free PMC article.
-
Lateral excitation within the olfactory bulb.J Neurosci. 2006 Feb 22;26(8):2269-77. doi: 10.1523/JNEUROSCI.4791-05.2006. J Neurosci. 2006. PMID: 16495454 Free PMC article.
-
The giant escape neurons of crayfish: Past discoveries and present opportunities.Front Physiol. 2022 Dec 20;13:1052354. doi: 10.3389/fphys.2022.1052354. eCollection 2022. Front Physiol. 2022. PMID: 36605900 Free PMC article. Review.
-
Effects of Ethanol on Sensory Inputs to the Medial Giant Interneurons of Crayfish.Front Physiol. 2018 Apr 27;9:448. doi: 10.3389/fphys.2018.00448. eCollection 2018. Front Physiol. 2018. PMID: 29755370 Free PMC article.
References
-
- Antonsen BL, Edwards DH (2003) Differential dye coupling reveals lateral giant escape circuit in crayfish. J Comp Neurol 466: 1-13. - PubMed
-
- Araki M, Nagayama T (2003) Direct chemically mediated synaptic transmission from mechanosensory afferents contributes to habituation of crayfish lateral giant escape reaction. J Comp Physiol [A] 189: 731-739. - PubMed
-
- Calabrese RL (1976) Crayfish mechanoreceptive interneurons. Part 1. The nature of ipsilateral excitatory inputs. J Comp Physiol [A] 105: 83-102.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
