Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo
- PMID: 15790660
- PMCID: PMC1464517
- DOI: 10.1113/jphysiol.2005.085506
Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo
Abstract
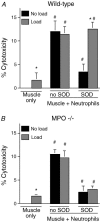
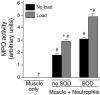
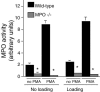
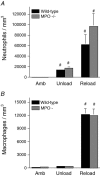
Membrane lysis is a common and early defect in muscles experiencing acute injuries or inflammation. Although increased mechanical loading of muscles can induce inflammation and membrane lysis, whether mechanical loads applied to muscle can promote the activation and cytolytic capacity of inflammatory cells and thereby increase muscle damage is unknown. We tested whether mechanical loads applied to mouse muscle cells in vitro can increase membrane lysis, and whether neutrophil-mediated lysis of muscle cells is promoted by mechanical loads applied in vitro and in vivo. Cyclic loads applied to muscle cells for 24 h in vitro produced little muscle cell lysis. Similarly, the addition of neutrophils to muscle cell cultures in the presence of superoxide dismutase (SOD) produced little muscle cell lysis. However, when cyclic mechanical loads were applied to neutrophil-muscle co-cultures in the presence of SOD, there was a synergistic effect on muscle cell lysis, suggesting that mechanical loading activates neutrophil cytotoxicity. However, application of mechanical loads to co-cultures of muscle cells and neutrophils that are null mutants for myeloperoxidase (MPO) showed no mechanical activation of neutrophil cytotoxicity. This indicates that loading promotes neutrophil cytotoxicity via MPO. Activity assays confirmed that mechanical loading of neutrophil-muscle co-cultures significantly increased MPO activity. We further tested whether muscle membrane lysis in vivo was mediated by neutrophils when muscle was subjected to modified loading by using a mouse model of muscle reloading following a period of unloading. We observed that MPO-/-soleus muscles showed a significant 52% reduction in membrane lysis compared to wild-type mice, although the mutation did not decrease inflammatory cell extravasation. Together, these in vitro and in vivo findings show that mechanical loading activates neutrophil-mediated lysis of muscle cells through an MPO-dependent pathway.
Figures




References
-
- Alessio HM, Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol. 1988;64:1333–1336. - PubMed
-
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. - PubMed
-
- Belcastro AN, Arthur GD, Albisser TA, Raj DA. Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J Appl Physiol. 1996;80:1331–1335. - PubMed
-
- Brennan ML, Gaur A, Pahuja A, Lusis AJ, Reynolds WF. Mice lacking myeloperoxidase are more susceptible to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001b;112:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

