Angiotensin II regulation of ovine fetoplacental artery endothelial functions: interactions with nitric oxide
- PMID: 15790666
- PMCID: PMC1464493
- DOI: 10.1113/jphysiol.2004.082420
Angiotensin II regulation of ovine fetoplacental artery endothelial functions: interactions with nitric oxide
Abstract
During normal pregnancy, elevated angiotensin II (Ang II) concentrations in the maternal and fetal circulations are associated with dramatic increases in placental angiogenesis and blood flow. Much is known about a local renin-angiotensin system within the uteroplacental vasculature. However, the roles of Ang II in regulating fetoplacental vascular functions are less well defined. In the fetal placenta, the overall in vivo vasoconstrictor responses of the blood vessels to Ang II infusion is thought to be less than that in its maternal counterpart, even though infused Ang II induces vasoconstriction. Recent data from our laboratories suggest that Ang II stimulates cell proliferation and increases endothelial nitric oxide synthase (eNOS) and production of nitric oxide (NO) in ovine fetoplacental artery endothelial cells. These data imply that elevations of the known vasoconstrictor Ang II in the fetal circulation may indeed play a role in the marked increases in fetoplacental angiogenesis and that Ang II-elevated endothelial NO production may partly attenuate Ang II-induced vasoconstriction on vascular smooth muscle. Together with both of these processes, the high levels of Ang II in the fetal circulation may serve to modulate overall fetoplacental vascular resistance. In this article, we review currently available data on the expression of Ang II receptors in the ovine fetal placenta with particular emphasis on the effects of Ang II on ovine fetoplacental endothelium. The potential cellular mechanisms underlying the regulation of Ang II on endothelial growth and vasodilator production are discussed.
Figures

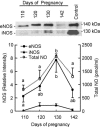
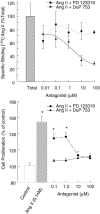
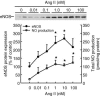

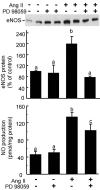
Comment in
-
Molecular, cellular and endocrine signalling in the perinatal cardiovascular system: interplay and developmental programming.J Physiol. 2005 May 15;565(Pt 1):1. doi: 10.1113/jphysiol.2005.086744. Epub 2005 Mar 24. J Physiol. 2005. PMID: 15790658 Free PMC article. No abstract available.
Similar articles
-
Angiotensin II elevates nitric oxide synthase 3 expression and nitric oxide production via a mitogen-activated protein kinase cascade in ovine fetoplacental artery endothelial cells.Biol Reprod. 2005 Jun;72(6):1421-8. doi: 10.1095/biolreprod.104.039172. Epub 2005 Feb 23. Biol Reprod. 2005. PMID: 15728793
-
Effects of pulsatile shear stress on nitric oxide production and endothelial cell nitric oxide synthase expression by ovine fetoplacental artery endothelial cells.Biol Reprod. 2003 Sep;69(3):1053-9. doi: 10.1095/biolreprod.102.013474. Epub 2003 May 28. Biol Reprod. 2003. PMID: 12773424
-
Effects of pulsatile shear stress on signaling mechanisms controlling nitric oxide production, endothelial nitric oxide synthase phosphorylation, and expression in ovine fetoplacental artery endothelial cells.Endothelium. 2005 Jan-Apr;12(1-2):21-39. doi: 10.1080/10623320590933743. Endothelium. 2005. PMID: 16036314
-
Angiotensin II and nitric oxide: a question of balance.Regul Pept. 1999 May 31;81(1-3):1-10. doi: 10.1016/s0167-0115(99)00027-0. Regul Pept. 1999. PMID: 10395403 Review.
-
Oxygen sensitivity, potassium channels, and regulation of placental vascular tone.Microcirculation. 2014 Jan;21(1):58-66. doi: 10.1111/micc.12069. Microcirculation. 2014. PMID: 23710683 Review.
Cited by
-
Activation of AP-1 transcription factors differentiates FGF2 and vascular endothelial growth factor regulation of endothelial nitric-oxide synthase expression in placental artery endothelial cells.J Biol Chem. 2010 Jun 4;285(23):17348-58. doi: 10.1074/jbc.M109.092791. Epub 2010 Apr 6. J Biol Chem. 2010. PMID: 20371606 Free PMC article.
-
Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways.Placenta. 2009 Dec;30(12):1045-51. doi: 10.1016/j.placenta.2009.10.007. Epub 2009 Nov 5. Placenta. 2009. PMID: 19892399 Free PMC article.
-
Estrogen Receptors and Estrogen-Induced Uterine Vasodilation in Pregnancy.Int J Mol Sci. 2020 Jun 18;21(12):4349. doi: 10.3390/ijms21124349. Int J Mol Sci. 2020. PMID: 32570961 Free PMC article. Review.
-
Signaling regulation of fetoplacental angiogenesis.J Endocrinol. 2012 Mar;212(3):243-55. doi: 10.1530/JOE-11-0296. Epub 2011 Nov 21. J Endocrinol. 2012. PMID: 22106098 Free PMC article. Review.
-
Transcriptomics analysis of the bovine endometrium during the perioestrus period.PLoS One. 2024 Mar 28;19(3):e0301005. doi: 10.1371/journal.pone.0301005. eCollection 2024. PLoS One. 2024. PMID: 38547106 Free PMC article.
References
-
- Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am J Physiol. 1990;259:C484–C489. - PubMed
-
- Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AH. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. - PubMed
-
- Bayraktutan U. Effects of angiotensin II on nitric oxide generation in growing and resting rat aortic endothelial cells. J Hypertens. 2003;21:2093–2101. - PubMed
-
- Bing C, Johnson IR, Broughton Pipkin F. Angiotensin receptors in myometrium and myometrial vessels from uteri of women during the follicular and luteal phases of the menstrual cycle and in late pregnancy. Clin Sci (Lond) 1996;90:499–505. - PubMed
-
- Bird IM, Mason JI, Rainey WE. Hormonal regulation of angiotensin II type 1 receptor expression and AT1-R mRNA levels in human adrenocortical cells. Endo Res. 1995;21:169–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL049210/HL/NHLBI NIH HHS/United States
- HD38843S/HD/NICHD NIH HHS/United States
- HL49210/HL/NHLBI NIH HHS/United States
- HL57653/HL/NHLBI NIH HHS/United States
- R01 HL074947/HL/NHLBI NIH HHS/United States
- R01 HL070562/HL/NHLBI NIH HHS/United States
- R01 HD033255/HD/NICHD NIH HHS/United States
- HD33255/HD/NICHD NIH HHS/United States
- HL64703/HL/NHLBI NIH HHS/United States
- R01 HL064703/HL/NHLBI NIH HHS/United States
- HL70562/HL/NHLBI NIH HHS/United States
- HL74947/HL/NHLBI NIH HHS/United States
- P01 HD038843/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

