Adult human hematopoietic stem cells produce neurons efficiently in the regenerating chicken embryo spinal cord
- PMID: 15790679
- PMCID: PMC556004
- DOI: 10.1073/pnas.0501029102
Adult human hematopoietic stem cells produce neurons efficiently in the regenerating chicken embryo spinal cord
Abstract
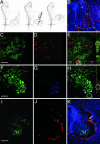
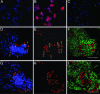
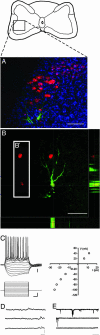
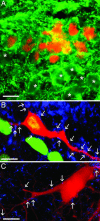
Hematopoietic stem cells (HSCs) have been proposed as a potential source of neural cells for use in repairing brain lesions, but previous studies indicate a low rate of neuronal differentiation and have not provided definite evidence of neuronal phenotype. To test the neurogenic potential of human HSCs, we implanted CD34+ HSCs from adult human bone marrow into lesions of the developing spinal cord in the chicken embryo and followed their differentiation by using immunohistochemistry, retrograde labeling, and electrophysiology. We find that human cells derived from the implanted population express the neuronal markers NeuN and MAP2 at substantially higher rates than previously reported. We also find that these cells exhibit neuronal cytoarchitecture, extend axons into the ventral roots or several segments in length within the spinal white matter, are decorated with synaptotagmin+ and GABA+ synaptic terminals, and exhibit active membrane properties and spontaneous synaptic potentials characteristic of functionally integrated neurons. Neuronal differentiation is accompanied by loss of CD34 expression. Careful examination with confocal microscopy reveals no signs of heterokaryons, and human cells never express a chicken-specific antigen, suggesting that fusion with host chicken cells is unlikely. We conclude that the microenvironment in the regenerating spinal cord of the chicken embryo stimulates substantial proportions of adult human HSCs to differentiate into full-fledged neurons. This may open new possibilities for a high-yield production of neurons from a patient's own bone marrow.
Figures

References
-
- Sanchez-Ramos, J., Song, S., Cardozo-Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T. B., Saporta, S., Janssen, W., Patel, N., et al. (2000) Exp. Neurol. 164, 247–256. - PubMed
-
- Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. (2000) Science 290, 1779–1782. - PubMed
-
- Song, H. J., Stevens, C. F. & Gage, F. H. (2002) Nat. Neurosci. 5, 438–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

