Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells
- PMID: 15790681
- PMCID: PMC555574
- DOI: 10.1073/pnas.0501675102
Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells
Abstract
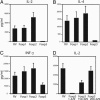
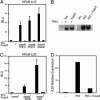
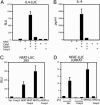
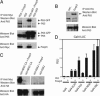
Scurfy mice, which are deficient in a functional Foxp3, exhibit a severe lymphoproliferative disorder and display generalized over-production of cytokines. Here, we show that, among the Foxp transcriptional factor family, which includes Foxp1, Foxp2, and Foxp3, only Foxp3 has the ability to inhibit IL-2, IL-4, and IFN-gamma production by primary T helper cells. We found that Foxp3 physically associates with the Rel family transcription factors, nuclear factor of activated T cells (NFAT) and NF-kappaB, and blocks their ability to induce the endogenous expression of their target genes, including key cytokine genes. More importantly, T cells derived from scurfy mice have a dramatic increase in nuclear factor of activated T cells (NFAT) and NF-kappa B transcriptional activity compared with the T cells derived from WT mice. Furthermore, complementation of Foxp3 in scurfy-derived T cells lowers the NFAT and NF-kappa B transcriptional activity to the physiological level. Finally, we show that myelin proteolipid protein-specific autoreactive T cells transduced with Foxp3 cannot mediate experimental autoimmune encephalomyelitis, providing further support that Foxp3 suppresses the effector function of autoreactive T cells. Foxp3 has already been associated with the generation of CD4(+)CD25+ regulatory T cells; our data additionally demonstrate that Foxp3 suppresses the effector functions of T helper cells by directly inhibiting the activity of two key transcription factors, NFAT and NF-kappa B, which are essential for cytokine gene expression and T cell functions.
Figures

References
-
- Kaufmann, E. & Knochel, W. (1996) Mech. Dev. 57, 3–20. - PubMed
-
- Carlsson, P. & Mahlapuu, M. (2002) Dev. Biol. 250, 1–23. - PubMed
-
- Banerjee-Basu, S. & Baxevanis, A. D. (2004) Proteins 54, 639–647. - PubMed
-
- Banham, A. H., Beasley, N., Campo, E., Fernandez, P. L., Fidler, C., Gatter, K., Jones, M., Mason, D. Y., Prime, J. E., Trougouboff, P., et al. (2001) Cancer Res. 61, 8820–8829. - PubMed
-
- Barrans, S. L., Fenton, J. A., Banham, A., Owen, R. G. & Jack, A. S. (2004) Blood 104, 2933–2935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

