Correlation between vesicle quantal size and fusion pore release in chromaffin cell exocytosis
- PMID: 15792983
- PMCID: PMC1305668
- DOI: 10.1529/biophysj.104.053736
Correlation between vesicle quantal size and fusion pore release in chromaffin cell exocytosis
Abstract
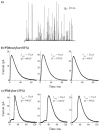
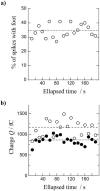
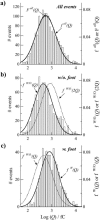
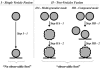
A significant number of exocytosis events recorded with amperometry demonstrate a prespike feature termed a "foot" and this foot has been correlated with messengers released via a transitory fusion pore before full exocytosis. We have compared amperometric spikes with a foot with spikes without a foot at chromaffin cells and found that the probability of detecting a distinct foot event is correlated to the amount of catecholamine released. The mean charge of the spikes with a foot was found to be twice that of the spikes without a foot, and the frequency of spikes displaying a foot was zero for small spikes increasing to approximately 50% for large spikes. It is hypothesized that in chromaffin cells, where the dense core is believed to nearly fill the vesicle, the expanding core is a controlling factor in opening the fusion pore, that prefusion of two smaller vesicles leads to excess membrane, and that this slows pore expansion leading to an increased observation of events with a foot. Clearly, the physicochemical properties of vesicles are key factors in the control of the dynamics of release through the fusion pore and the high and variable frequency of this release makes it highly significant.
Figures
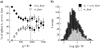
References
-
- Albillos, A., G. Dernick, H. Horstmann, W. Almers, G. A. deToledo, and M. Lindau. 1997. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 389:509–512. - PubMed
-
- Almers, W. 2001. Fusion needs more than SNAREs. Nature. 409:567–568. - PubMed
-
- Amatore, C., Y. Bouret, and L. Midrier. 1999. Time-resolved dynamics of the vesicle membrane during individual exocytotic secretion events, as extracted from amperometric monitoring of adrenaline exocytosis from chromaffin cells. Biochimie. 5:2151–2162.
-
- Amatore, C., Y. Bouret, E. R. Travis, and R. M. Wightman. 2000a. Adrenaline release by chromaffin cells: Constrained swelling of the vesicle matrix leads to full fusion. Angew. Chem. Int. Ed. 39:1952–1955. - PubMed
-
- Amatore, C., Y. Bouret, E. R. Travis, and R. M. Wightman. 2000b. Interplay between membrane dynamics, diffusion and swelling pressure governs individual vesicular exocytotic events during release of adrenaline by chromaffin cells. Biochimie. 82:481–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

