Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing
- PMID: 15793001
- PMCID: PMC555605
- DOI: 10.1073/pnas.0501463102
Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing
Abstract
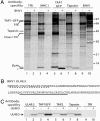
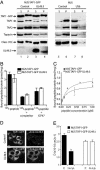
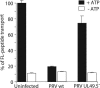
Detection and elimination of virus-infected cells by cytotoxic T lymphocytes depends on recognition of virus-derived peptides presented by MHC class I molecules. A critical step in this process is the translocation of peptides from the cytoplasm into the endoplasmic reticulum by the transporter associated with antigen processing (TAP). Here, we identified the bovine herpesvirus 1-encoded UL49.5 protein as a potent inhibitor of TAP. The expression of UL49.5 results in down-regulation of MHC class I molecules at the cell surface and inhibits detection and lysis of the cells by cytotoxic T lymphocytes. UL49.5 homologs encoded by two other varicelloviruses, pseudorabies-virus and equine herpesvirus 1, also block TAP. Homologs of UL49.5 are widely present in herpesviruses, acting as interaction partners for glycoprotein M, but in several varicelloviruses UL49.5 has uniquely evolved additional functions that mediate its participation in TAP inhibition. Inactivation of TAP by UL49.5 involves two events: inhibition of peptide transport through a conformational arrest of the transporter and degradation of TAP by proteasomes. UL49.5 is degraded along with TAP via a reaction that requires the cytoplasmic tail of UL49.5. Thus, UL49.5 represents a unique immune evasion protein that inactivates TAP through a unique two-tiered process.
Figures
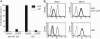



References
-
- Tortorella, D., Gewurz, B. E., Furman, M. H., Schust, D. J. & Ploegh, H. L. (2000) Annu. Rev. Immunol. 18, 861–926. - PubMed
-
- Yewdell, J. W. & Hill, A. B. (2002) Nat. Immunol. 3, 1019–1025. - PubMed
-
- Cresswell, P. (2000) Traffic 4, 301–305. - PubMed
-
- Sadasivan, B., Lehner, P. J., Ortmann, B., Spies, T. & Cresswell, P. (1996) Immunity 5, 103–114. - PubMed
-
- Ortmann, B., Copeman, J., Lehner, P. J., Sadasivan, B., Herberg, J. A., Grandea, A. G., Riddell, S. R., Tampe, R., Spies, T., Trowsdale, J. & Cresswell, P. (1997) Science 277, 1306–1309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

