A protein folding pathway with multiple folding intermediates at atomic resolution
- PMID: 15793003
- PMCID: PMC555603
- DOI: 10.1073/pnas.0501372102
A protein folding pathway with multiple folding intermediates at atomic resolution
Abstract
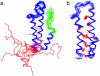
Using native-state hydrogen-exchange-directed protein engineering and multidimensional NMR, we determined the high-resolution structure (rms deviation, 1.1 angstroms) for an intermediate of the four-helix bundle protein: Rd-apocytochrome b562. The intermediate has the N-terminal helix and a part of the C-terminal helix unfolded. In earlier studies, we also solved the structures of two other folding intermediates for the same protein: one with the N-terminal helix alone unfolded and the other with a reorganized hydrophobic core. Together, these structures provide a description of a protein folding pathway with multiple intermediates at atomic resolution. The two general features for the intermediates are (i) native-like backbone topology and (ii) nonnative side-chain interactions. These results have implications for important issues in protein folding studies, including large-scale conformation search, -value analysis, and computer simulations.
Figures



References
-
- Kim, P. S. & Baldwin, R. L. (1982) Annu. Rev. Biochem. 51, 459–489. - PubMed
-
- Kim, P. S. & Baldwin, R. L. (1990) Annu. Rev. Biochem. 59, 631–660. - PubMed
-
- Englander, S. W. & Mayne, L. (1992) Annu. Rev. Biophys. Biomol. Struct. 21, 243–265. - PubMed
-
- Matthews, C. R. (1993) Annu. Rev. Biochem. 62, 653–683. - PubMed
-
- Ptitsyn, O. B. (1995) Curr. Opin. Struct. Biol. 5, 74–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

