Development of cell-based assays for in vitro characterization of hepatitis C virus NS3/4A protease inhibitors
- PMID: 15793116
- PMCID: PMC1068595
- DOI: 10.1128/AAC.49.4.1381-1390.2005
Development of cell-based assays for in vitro characterization of hepatitis C virus NS3/4A protease inhibitors
Abstract
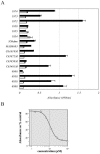
A recombinant vaccinia virus, expressing the NS3-to-NS5 region of the N clone of hepatitis C virus (HCV), was generated and utilized both in a gel-based assay and in an enzyme-linked immunosorbent assay (ELISA) to evaluate the pyrrolidine-5,5-trans-lactams, a series of inhibitors of the HCV NS3/4A protease. The absolute levels of processed, mature HCV nonstructural proteins in this system were found to decrease in the presence of the trans-lactams. Monitoring of this reduction enabled end points and 50% inhibitory concentrations to be calculated in order to rank the active compounds according to potency. These compounds had no effect on the transcription or translation of the NS3-5 polyprotein at concentrations shown to inhibit NS3/4A protease, and they were shown to be specific inhibitors of this protease. The ELISA, originally developed using the vaccinia virus expression system, was modified to utilize Huh-7 cells containing an HCV replicon. Results with this assay correlated well with those obtained with the recombinant vaccinia virus assays. These results demonstrate the utility of these assays for the characterization of NS3/4A protease inhibitors. In addition, inhibitors of other viral targets, such as polymerase and helicase, can be evaluated in the context of the replicon ELISA.
Figures



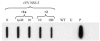



References
-
- Andrews, D. M., S. J. Carey, H. Chaignot, B. A. Coomber, N. M. Gray, S. L. Hind, P. S. Jones, G. Mills, J. E. Robinson, and M. J. Slater. 2002. Pyrrolidine-5,5-trans-lactams. 1. Synthesis and incorporation into inhibitors of hepatitis C virus NS3/4A protease. Org. Lett. 4:4475-4478. - PubMed
-
- Andrews, D. M., H. M. Chaignot, B. A. Coomber, A. C. Good, S. L. Hind, M. R. Johnson, P. S. Jones, G. Mills, J. E. Robinson, T. Skarzynski, M. J. Slater, and D. O. Somers. 2002. Pyrrolidine-5,5-trans-lactams. 2. The use of X-ray crystal structure data in the optimisation of P3 and P4 substituents. Org. Lett. 4:4479-4482. - PubMed
-
- Andrews, D. M., P. S. Jones, G. Mills, S. L. Hind, M. J. Slater, N. Trivedi, and K. J. Wareing. 2003. Design and synthesis of spiro-cyclopentenyl and spiro-[1,3]-dithiolanyl substituted pyrrolidine-5,5-trans-lactams as inhibitors of hepatitis C virus NS3/4A protease. Bioorg. Med. Chem. Lett. 13:1657-1660. - PubMed
-
- Andrews, D. M., H. M. Chaignot, B. A. Coomber, M. D. Dowle, S. L. Hind, M. R. Johnson, P. S. Jones, G. Mills, A. Patikis, T. J. Pateman, J. E. Robinson, M. J. Slater, and N. Trivedi. 2003. The design of potent, non-peptidic inhibitors of hepatitis C protease. Eur. J. Med. Chem. 38:339-343. - PubMed
-
- Andrews, D. M., M. C. Barnes, M. D. Dowle, S. L. Hind, M. R. Johnson, P. S. Jones, G. Mills, A. Patikis, T. J. Pateman, T. J. Redfern, J. E. Robinson, M. J. Slater, and N. Trivedi. 2003. Pyrrolidine-5,5-trans-lactams. 5. Pharmacokinetic optimisation of inhibitors of hepatitis C virus NS3/4A protease. Org. Lett. 5:4631-4634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

