Type I interferon dependence of plasmacytoid dendritic cell activation and migration
- PMID: 15795237
- PMCID: PMC2213121
- DOI: 10.1084/jem.20041930
Type I interferon dependence of plasmacytoid dendritic cell activation and migration
Abstract
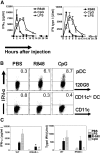
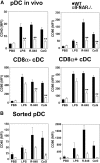
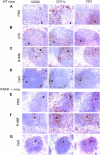
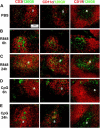
Differential expression of Toll-like receptor (TLR) by conventional dendritic cells (cDCs) and plasmacytoid DC (pDCs) has been suggested to influence the type of immune response induced by microbial pathogens. In this study we show that, in vivo, cDCs and pDCs are equally activated by TLR4, -7, and -9 ligands. Type I interferon (IFN) was important for pDC activation in vivo in response to all three TLR ligands, whereas cDCs required type I IFN signaling only for TLR9- and partially for TLR7-mediated activation. Although TLR ligands induced in situ migration of spleen cDC into the T cell area, spleen pDCs formed clusters in the marginal zone and in the outer T cell area 6 h after injection of TLR9 and TLR7 ligands, respectively. In vivo treatment with TLR9 ligands decreased pDC ability to migrate ex vivo in response to IFN-induced CXCR3 ligands and increased their response to CCR7 ligands. Unlike cDCs, the migration pattern of pDCs required type I IFN for induction of CXCR3 ligands and responsiveness to CCR7 ligands. These data demonstrate that mouse pDCs differ from cDCs in the in vivo response to TLR ligands, in terms of pattern and type I IFN requirement for activation and migration.
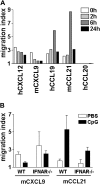
Figures


References
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. - PubMed
-
- Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. - PubMed
-
- Sallusto, F., and A. Lanzavecchia. 2000. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177:134–140. - PubMed
-
- Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

