Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis
- PMID: 15795262
- PMCID: PMC1069551
- DOI: 10.1128/JVI.79.8.4764-4773.2005
Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis
Abstract
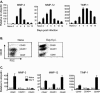
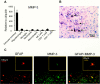
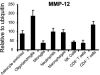
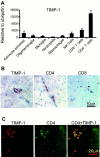
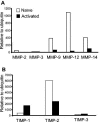
Matrix metalloproteinases (MMPs) participate in remodeling the extracellular matrix and facilitate entry of inflammatory cells into tissues. Infection of the murine central nervous system (CNS) with a neurotropic coronavirus induces encephalitis associated with increased levels of mRNA encoding MMP-3 and MMP-12. Whereas virus-induced MMP-3 expression was restricted to CNS resident astrocytes, MMP-12 mRNA was expressed by both inflammatory cells and CNS resident cells. Immunosuppression increased both MMP-3 and MMP-12 mRNA levels in CNS resident cells, suggesting that the presence of virus rather than inflammation induced protease up-regulation. MMP activity is partially regulated by a small family of genes encoding tissue inhibitors of matrix metalloproteinases (TIMPs); among the TIMPs, only TIMP-1 mRNA expression increased in the CNS following coronavirus infection. During inflammation TIMP-1 mRNA was most prominently expressed by infiltrating cells. By contrast, in the immunosuppressed host TIMP-1 mRNA was expressed by CNS resident cells. Analysis of cytokine and chemokine mRNA induction within the infected CNS of healthy and immunocompromised mice suggested a possible correlation between increased viral replication and increased levels of beta interferon, MMP-3, MMP-12, and TIMP-1 mRNA. CD4+ T cells which localize to the perivascular and subarachnoid spaces were identified as the primary source of TIMP-1 protein. By contrast, protein expression was undetectable in astrocytes or CD8+ T cells, the primary antiviral effectors that localize to the CNS parenchyma in response to infection. These data suggest that in contrast to the results seen with MMPs, inhibition of protease activity via TIMP-1 expression correlates with the differential tissue distribution of T-cell subsets during acute coronavirus-induced encephalitis.
Figures


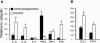
References
-
- Alter, A., M. Duddy, S. Herbert, K. Biernacki, A. Prtat, J. Antel, V. W. Yong, R. K. Nuttall, C. J. Pennington, D. R. Edwards, and A. Bar-Or. 2003. Determinants of human B cell migration across brain endothelial cells. J. Immunol. 170:4497-4505. - PubMed
-
- Bart, J., H. J. Groen, N. K. Hendrikse, W.T. van der Graaf, W. Vaalburg, and E. G. de Vries. 2000. The blood-brain barrier and oncology: new insights into function and modulation. Cancer Treat. Rev. 26:449-462. - PubMed
-
- Bergmann, C., B. Parra, D. R. Hinton, C. Ramakrishna. M. Morrison, and S. A. Stohlman. 2003. Perforin mediated effector function within the CNS requires IFN-g mediated MHC upregulation. J. Immunol. 170:3204-3213. - PubMed
-
- Brosnan, C. F., W. Cammer, W. T. Norton, and R. Bloom. 1980. Proteinase inhibitors suppress the development of experimental allergic encephalomyelitis. Nature 285:235-237. - PubMed
-
- Bugnu, M., B. Witek, J. Bereta, M. Bereta, D. R. Edwards, and T. Kordula. 1999. Reprogramming of TIMP-1 and TIMP-3 expression profiles in brain microvascular endothelial cells and astrocytes in response to proinflammatory cytokines. FEBS Lett. 448:9-14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

