Escape mutations alter proteasome processing of major histocompatibility complex class I-restricted epitopes in persistent hepatitis C virus infection
- PMID: 15795272
- PMCID: PMC1069526
- DOI: 10.1128/JVI.79.8.4870-4876.2005
Escape mutations alter proteasome processing of major histocompatibility complex class I-restricted epitopes in persistent hepatitis C virus infection
Abstract
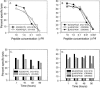
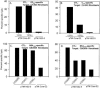
Mutations in hepatitis C virus (HCV) genomes facilitate escape from virus-specific CD8+ T lymphocytes in persistently infected chimpanzees. Our previous studies demonstrated that many of the amino acid substitutions in HCV epitopes prevented T-cell receptor recognition or binding to class I major histocompatibility complex molecules. Here we report that mutations within HCV epitopes also cause their destruction by changing the pattern of proteasome digestion. This mechanism of immune evasion provides further evidence of the potency of CD8+ T-cell selection pressure against HCV and should be considered when evaluating the significance of mutations in viral genomes from persistently infected chimpanzees and humans.
Figures



References
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. - PubMed
-
- Beekman, N. J., P. A. van Veelen, T. van Hall, A. Neisig, A. Sijts, M. Camps, P. M. Kloetzel, J. J. Neefjes, C. J. Melief, and F. Ossendorp. 2000. Abrogation of CTL epitope processing by single amino acid substitution flanking the C-terminal proteasome cleavage site. J. Immunol. 164:1898-1905. - PubMed
-
- Cardozo, C., and R. A. Kohanski. 1998. Altered properties of the branched chain amino acid-preferring activity contribute to increased cleavages after branched chain residues by the “immunoproteasome.” J. Biol. Chem. 273:16764-16770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

