Comparison of inflammatory and acute-phase responses in the brain and peripheral organs of the ME7 model of prion disease
- PMID: 15795301
- PMCID: PMC1069550
- DOI: 10.1128/JVI.79.8.5174-5184.2005
Comparison of inflammatory and acute-phase responses in the brain and peripheral organs of the ME7 model of prion disease
Abstract
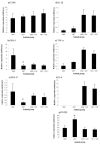
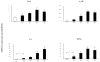
Chronic neurodegenerative diseases such as prion disease and Alzheimer's disease (AD) are reported to be associated with microglial activation and increased brain and serum cytokines and acute-phase proteins (APPs). Unlike AD, prion disease is also associated with a peripheral component in that the presumed causative agent, PrPSc, also accumulates in the spleen and other lymphoreticular organs. It is unclear whether the reported systemic acute-phase response represents a systemic inflammatory response to prion disease or merely reflects central nervous system (CNS) inflammation. For this study, we investigated whether intracerebrally initiated prion disease (ME7 model) provokes splenic, hepatic, or brain inflammatory and acute-phase responses. We detected no significant elevation of proinflammatory cytokines or activation of macrophages in the spleens of these animals, despite clear PrPSc deposition. Similarly, at 19 weeks we detected no significant elevation of transcripts for the APPs serum amyloid A, complement C3, pentraxin 3, and alpha2-antiplasmin in the liver, despite CNS neurodegeneration and splenic PrPSc deposition at this time. However, despite the low CNS expression levels of proinflammatory cytokines, there was robust expression of these APPs in degenerating brains. These findings suggest that PrPSc is not a stimulus for splenic macrophages and that neither peripheral PrPSc deposition nor CNS neurodegeneration is sufficient to produce a systemic acute-phase response. We also propose that serum cytokine and APP measurements are not useful during preclinical disease. Possible consequences of the clear chronic elevation of APPs in the CNS are discussed.
Figures

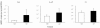
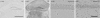
References
-
- Arai, H., V. M. Lee, M. L. Messinger, B. D. Greenberg, D. E. Lowery, and J. Q. Trojanowski. 1991. Expression patterns of beta-amyloid precursor protein (beta-APP) in neural and nonneural human tissues from Alzheimer's disease and control subjects. Ann. Neurol. 30:686-693. - PubMed
-
- Argmann, C. A., C. H. Van Den Diepstraten, C. G. Sawyez, J. Y. Edwards, R. A. Hegele, B. M. Wolfe, and M. W. Huff. 2001. Transforming growth factor-beta1 inhibits macrophage cholesteryl ester accumulation induced by native and oxidized VLDL remnants. Arterioscler. Thromb. Vasc. Biol. 21:2011-2018. - PubMed
-
- Betmouni, S., and V. H. Perry. 1999. The acute inflammatory response in CNS following injection of prion brain homogenate or normal brain homogenate. Neuropathol. Appl. Neurobiol. 25:20-28. - PubMed
-
- Betmouni, S., V. H. Perry, and J. L. Gordon. 1996. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience 74:1-5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

