Tissue-resident macrophages are productively infected ex vivo by primary X4 isolates of human immunodeficiency virus type 1
- PMID: 15795306
- PMCID: PMC1069582
- DOI: 10.1128/JVI.79.8.5220-5226.2005
Tissue-resident macrophages are productively infected ex vivo by primary X4 isolates of human immunodeficiency virus type 1
Abstract
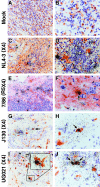
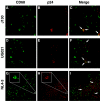
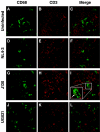
Infection of macrophages has been implicated as a critical event in the transmission and persistence of human immunodeficiency virus type 1 (HIV-1). Here, we explore whether primary X4 HIV-1 isolates can productively infect tissue macrophages that have terminally differentiated in vivo. Using immunohistochemistry, HIV-1 RNA in situ hybridization, and confocal immunofluorescence microscopy, we demonstrate that macrophages residing in human tonsil blocks can be productively infected ex vivo by primary X4 HIV-1 isolates. This challenges the model in which macrophage tropism is a key determinant of the selective transmission of R5 HIV-1 strains. Infection of tissue macrophages by X4 HIV-1 may be highly relevant in vivo and contribute to key events in HIV-1 pathogenesis.
Figures

Similar articles
-
Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism.J Virol. 1999 Sep;73(9):7117-25. doi: 10.1128/JVI.73.9.7117-7125.1999. J Virol. 1999. PMID: 10438797 Free PMC article.
-
Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway.J Virol. 2002 Sep;76(18):9407-19. doi: 10.1128/jvi.76.18.9407-9419.2002. J Virol. 2002. PMID: 12186923 Free PMC article.
-
An unusual syncytia-inducing human immunodeficiency virus type 1 primary isolate from the central nervous system that is restricted to CXCR4, replicates efficiently in macrophages, and induces neuronal apoptosis.J Neurovirol. 2003 Aug;9(4):432-41. doi: 10.1080/13550280390218706. J Neurovirol. 2003. PMID: 12907388
-
The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages.Rev Med Virol. 2003 Jan-Feb;13(1):39-56. doi: 10.1002/rmv.369. Rev Med Virol. 2003. PMID: 12516061 Review.
-
The macrophage response to HIV-1: Intracellular control of X4 virus replication accompanied by activation of chemokine and cytokine synthesis.J Neurovirol. 2002 Dec;8(6):599-610. doi: 10.1080/13550280290100923. J Neurovirol. 2002. PMID: 12476353 Review.
Cited by
-
Activation-neutral gene editing of tonsillar CD4 T cells for functional studies in human ex vivo tonsil cultures.Cell Rep Methods. 2024 Jan 22;4(1):100685. doi: 10.1016/j.crmeth.2023.100685. Epub 2024 Jan 10. Cell Rep Methods. 2024. PMID: 38211593 Free PMC article.
-
Selective transmission of R5 HIV-1 variants: where is the gatekeeper?J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S6. doi: 10.1186/1479-5876-9-S1-S6. J Transl Med. 2011. PMID: 21284905 Free PMC article. Review.
-
HIV-1 Tat-induced disruption of epithelial junctions and epithelial-mesenchymal transition of oral and genital epithelial cells lead to increased invasiveness of neoplastic cells and the spread of herpes simplex virus and cytomegalovirus.Front Immunol. 2025 Feb 13;16:1541532. doi: 10.3389/fimmu.2025.1541532. eCollection 2025. Front Immunol. 2025. PMID: 40018040 Free PMC article. Review.
-
Macrophages: Key Cellular Players in HIV Infection and Pathogenesis.Viruses. 2024 Feb 13;16(2):288. doi: 10.3390/v16020288. Viruses. 2024. PMID: 38400063 Free PMC article. Review.
-
HIV-1 cell-to-cell spread overcomes the virus entry block of non-macrophage-tropic strains in macrophages.PLoS Pathog. 2022 May 27;18(5):e1010335. doi: 10.1371/journal.ppat.1010335. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35622876 Free PMC article.
References
-
- Adams, C. W., and R. N. Poston. 1990. Macrophage histology in paraffin-embedded multiple sclerosis plaques is demonstrated by the monoclonal pan-macrophage marker HAM-56: correlation with chronicity of the lesion. Acta Neuropathol. 80:208-211. - PubMed
-
- Autschbach, F., E. Palou, G. Mechtersheimer, C. Rohr, F. Pirotto, N. Gassler, H. F. Otto, B. Schraven, and A. Gaya. 1999. Expression of the membrane protein tyrosine phosphatase CD148 in human tissues. Tissue Antigens 54:485-498. - PubMed
-
- Collman, R., N. F. Hassan, R. Walker, B. Godfrey, J. Cutilli, J. C. Hastings, H. Friedman, S. D. Douglas, and N. Nathanson. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 170:1149-1163. - PMC - PubMed
-
- Collman, R. G., and Y. Yi. 1999. Cofactors for human immunodeficiency virus entry into primary macrophages. J. Infect. Dis. 179:S422-S426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

