Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine
- PMID: 15795311
- PMCID: PMC2217510
- DOI: 10.1085/jgp.200409242
Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine
Abstract
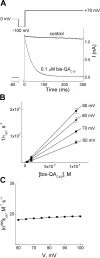
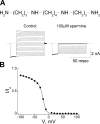
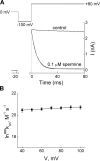
IRK1 (Kir2.1) inward-rectifier K+ channels exhibit exceedingly steep rectification, which reflects strong voltage dependence of channel block by intracellular cations such as the polyamine spermine. On the basis of studies of IRK1 block by various amine blockers, it was proposed that the observed voltage dependence (valence approximately 5) of IRK1 block by spermine results primarily from K+ ions, not spermine itself, traversing the transmembrane electrical field that drops mostly across the narrow ion selectivity filter, as spermine and K+ ions displace one another during channel block and unblock. If indeed spermine itself only rarely penetrates deep into the ion selectivity filter, then a long blocker with head groups much wider than the selectivity filter should exhibit comparably strong voltage dependence. We confirm here that channel block by two molecules of comparable length, decane-bis-trimethylammonium (bis-QA(C10)) and spermine, exhibit practically identical overall voltage dependence even though the head groups of the former are much wider ( approximately 6 A) than the ion selectivity filter ( approximately 3 A). For both blockers, the overall equilibrium dissociation constant differs from the ratio of apparent rate constants of channel unblock and block. Also, although steady-state IRK1 block by both cations is strongly voltage dependent, their apparent channel-blocking rate constant exhibits minimal voltage dependence, which suggests that the pore becomes blocked as soon as the blocker encounters the innermost K+ ion. These findings strongly suggest the existence of at least two (potentially identifiable) sequentially related blocked states with increasing numbers of K+ ions displaced. Consequently, the steady-state voltage dependence of IRK1 block by spermine or bis-QA(C10) should increase with membrane depolarization, a prediction indeed observed. Further kinetic analysis identifies two blocked states, and shows that most of the observed steady-state voltage dependence is associated with the transition between blocked states, consistent with the view that the mutual displacement of blocker and K+ ions must occur mainly as the blocker travels along the long inner pore.
Figures
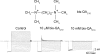






Similar articles
-
Evidence for sequential ion-binding loci along the inner pore of the IRK1 inward-rectifier K+ channel.J Gen Physiol. 2005 Aug;126(2):123-35. doi: 10.1085/jgp.200509296. J Gen Physiol. 2005. PMID: 16043774 Free PMC article.
-
Mechanism of rectification in inward-rectifier K+ channels.J Gen Physiol. 2003 Apr;121(4):261-75. doi: 10.1085/jgp.200208771. Epub 2003 Mar 17. J Gen Physiol. 2003. PMID: 12642596 Free PMC article.
-
Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels.J Gen Physiol. 2003 Nov;122(5):485-500. doi: 10.1085/jgp.200308890. J Gen Physiol. 2003. PMID: 14581581 Free PMC article.
-
Mechanism of rectification in inward-rectifier K+ channels.Annu Rev Physiol. 2004;66:103-29. doi: 10.1146/annurev.physiol.66.032102.150822. Annu Rev Physiol. 2004. PMID: 14977398 Review.
-
Polyamines as gating molecules of inward-rectifier K+ channels.Eur J Biochem. 2000 Oct;267(19):5824-9. doi: 10.1046/j.1432-1327.2000.01669.x. Eur J Biochem. 2000. PMID: 10998040 Review.
Cited by
-
Multi-ion distributions in the cytoplasmic domain of inward rectifier potassium channels.Biophys J. 2012 Aug 8;103(3):434-443. doi: 10.1016/j.bpj.2012.06.023. Biophys J. 2012. PMID: 22947859 Free PMC article.
-
LPS-induced CCL2 expression and macrophage influx into the murine central nervous system is polyamine-dependent.Brain Behav Immun. 2011 May;25(4):629-39. doi: 10.1016/j.bbi.2010.12.016. Epub 2011 Jan 13. Brain Behav Immun. 2011. PMID: 21237263 Free PMC article.
-
Intrinsic versus extrinsic voltage sensitivity of blocker interaction with an ion channel pore.J Gen Physiol. 2010 Feb;135(2):149-67. doi: 10.1085/jgp.200910324. J Gen Physiol. 2010. PMID: 20100894 Free PMC article.
-
The polyamine binding site in inward rectifier K+ channels.J Gen Physiol. 2006 May;127(5):467-80. doi: 10.1085/jgp.200509467. Epub 2006 Apr 10. J Gen Physiol. 2006. PMID: 16606689 Free PMC article.
-
Cardiac strong inward rectifier potassium channels.J Mol Cell Cardiol. 2010 Jan;48(1):45-54. doi: 10.1016/j.yjmcc.2009.08.013. Epub 2009 Aug 22. J Mol Cell Cardiol. 2010. PMID: 19703462 Free PMC article. Review.
References
-
- Ashcroft, F.M. 2000. Ion Channels and Disease: Channelopathies. Academic Press, San Diego. 481 pp.
-
- Carter, P.J., G. Winter, A.J. Wilkinson, and A.R. Fersht. 1984. The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus). Cell. 38:835–840. - PubMed
-
- Dibb, K.M., T. Rose, S.Y. Makary, T.W. Claydon, D. Enkvetchakul, R. Leach, C.G. Nichols, and M.R. Boyett. 2003. Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 channel. J. Biol. Chem. 278:49537–49548. - PubMed
-
- Fakler, B., U. Brandle, E. Glowatzki, S. Weidemann, H.P. Zenner, and J.P. Ruppersberg. 1995. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 80:149–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

