Comparative structure and pollen production of the stamens and pollinator-deceptive staminodes of Commelina coelestis and C. dianthifolia (Commelinaceae)
- PMID: 15797898
- PMCID: PMC4246906
- DOI: 10.1093/aob/mci134
Comparative structure and pollen production of the stamens and pollinator-deceptive staminodes of Commelina coelestis and C. dianthifolia (Commelinaceae)
Abstract
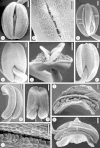
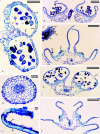
Background and aims: Flowers of Commelina coelestis and C. dianthifolia provide pollen alone as a floral reward, and rely on visual cues to attract pollinators. Three stamen types, all producing pollen, occur in each of these species: two cryptically coloured lateral stamens, a single cryptically coloured central stamen and three bright yellow staminodes that sharply contrast with the blue to purple corolla. The objective was to compare the stamen structure and pollen characteristics of each of the three stamen types, and to test the hypothesis that the staminodes are poor contributors of viable pollen for the siring of seed. The pollination roles of the three stamen types and the breeding systems of both species were also explored.
Methods: Light, fluorescence and scanning electron microscopy were utilized to examine stamen morphology and pollen structure and viability. Controlled hand pollinations were used to explore the breeding system of each species. Filament and style lengths were measured to investigate herkogamy and autogamy.
Key results: Pollen from all stamen morphs is viable, but staminode pollen has significantly lower viability. Pollen polymorphism exists both (a) between the lateral and central stamens and the staminodes, and (b) within each anther. Lateral and central stamens have thicker endothecia with a greater number of secondary cell wall thickenings than the staminodes.
Conclusions: Both species are entomophilous and facultatively autogamous. Lateral stamen pollen is important for cross-pollination, central stamen pollen is utilized by both species as a pollinator reward and for delayed autogamy in C. dianthifolia, and the staminodes mimic, by means of both colour and epidermal features, large amounts of pollen to attract insects to the flowers. Pollen from all three anther morphs is capable of siring seed, although staminode pollen is inferior. The thin staminode endothecium with fewer secondary thickenings retards staminode dehiscence.
Figures






Similar articles
-
In the right place at the right time: Parnassia resolves the herkogamy dilemma by accurate repositioning of stamens and stigmas.Ann Bot. 2014 Jan;113(1):97-103. doi: 10.1093/aob/mct261. Epub 2013 Nov 20. Ann Bot. 2014. PMID: 24265349 Free PMC article.
-
The evolution of staminodes in angiosperms: patterns of stamen reduction, loss, and functional re-invention.Am J Bot. 2000 Oct;87(10):1367-84. Am J Bot. 2000. PMID: 11034915
-
Development and structure of four different stamens in Clematis macropetala (Ranunculaceae): particular emphasis on staminodes and staminal nectary.Protoplasma. 2022 May;259(3):627-640. doi: 10.1007/s00709-021-01687-1. Epub 2021 Jul 11. Protoplasma. 2022. PMID: 34247271
-
Mechanisms and evolution of deceptive pollination in orchids.Biol Rev Camb Philos Soc. 2006 May;81(2):219-35. doi: 10.1017/S1464793105006986. Biol Rev Camb Philos Soc. 2006. PMID: 16677433 Review.
-
Pollen, anther, stamen, and androecium mimicry.Plant Biol (Stuttg). 2024 Apr;26(3):349-368. doi: 10.1111/plb.13628. Epub 2024 Feb 26. Plant Biol (Stuttg). 2024. PMID: 38407440 Review.
Cited by
-
Flower orientation enhances pollen transfer in bilaterally symmetrical flowers.Oecologia. 2009 Jul;160(4):667-74. doi: 10.1007/s00442-009-1334-9. Epub 2009 Apr 1. Oecologia. 2009. PMID: 19333624
-
Heteromorphism of stamens in monoclinous flowers of Tinantia erecta (Jacq.) Fenzl as an example of high variability of the androecium in the Commelinaceae family.Protoplasma. 2020 Sep;257(5):1473-1485. doi: 10.1007/s00709-020-01522-z. Epub 2020 Jun 26. Protoplasma. 2020. PMID: 32588232 Free PMC article.
-
Floral polymorphism in Chamaecrista flexuosa (Fabaceae-Caesalpinioideae): a possible case of atypical enantiostyly?Ann Bot. 2013 Oct;112(6):1117-23. doi: 10.1093/aob/mct188. Epub 2013 Sep 11. Ann Bot. 2013. PMID: 24026440 Free PMC article.
References
-
- Baker FJ, Silverton RE. 1976.Introduction to medical laboratory technology, 5th edn. London: Butterworths.
-
- Brantjes NBM. 1980. Individualisme bij bloembezoekende insekten op Commelina [Individualism by flower-seeking insects on Commelina]. De Levende Natuur 82: 9–16.
-
- Brashier CK. 1966. A revision of Commelina (Plum.) L. in the U.S.A. Bulletin of the Torrey Botanical Club 93: 1–19.
-
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50: 859–865.
-
- Christensen KI, Hansen HV. 1998. SEM-studies of epidermal patterns of petals in the angiosperms. Opera Botanica 135: 1–91.

