A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation
- PMID: 15798214
- PMCID: PMC1069628
- DOI: 10.1128/MCB.25.8.3305-3316.2005
A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation
Abstract
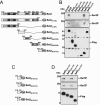
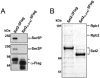
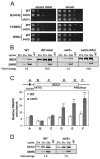
Histone methylation and the enzymes that mediate it are important regulators of chromatin structure and gene transcription. In particular, the histone H3 lysine 36 (K36) methyltransferase Set2 has recently been shown to associate with the phosphorylated C-terminal domain (CTD) of RNA polymerase II (RNAPII), implying that this enzyme has an important role in the transcription elongation process. Here we show that a novel domain in the C terminus of Set2 is responsible for interaction between Set2 and RNAPII. This domain, termed the Set2 Rpb1 interacting (SRI) domain, is encompassed by amino acid residues 619 to 718 in Set2 and is found to occur in a number of putative Set2 homologs from Schizosaccharomyces pombe to humans. Unexpectedly, BIACORE analysis reveals that the SRI domain binds specifically, and with high affinity, to CTD repeats that are doubly modified (serine 2 and serine 5 phosphorylated), indicating that Set2 association across the body of genes requires a specific pattern of phosphorylated RNAPII. Deletion of the SRI domain not only abolishes Set2-RNAPII interaction but also abolishes K36 methylation in vivo, indicating that this interaction is required for establishing K36 methylation on chromatin. Using 6-azauracil (6AU) as an indicator of transcription elongation defects, we found that deletion of the SRI domain conferred a strong resistance to this compound, which was identical to that observed with set2 deletion mutants. Furthermore, yeast strains carrying set2 alleles that are catalytically inactive or yeast strains bearing point mutations at K36 were also found to be resistant to 6AU. These data suggest that it is the methylation by Set2 that affects transcription elongation. In agreement with this, we have determined that deletion of SET2, its SRI domain, or amino acid substitutions at K36 result in an alteration of RNAPII occupancy levels over transcribing genes. Taken together, these data indicate K36 methylation, established by the SRI domain-mediated association of Set2 with RNAPII, plays an important role in the transcription elongation process.
Figures



References
-
- Ahn, S. H., M. Kim, and S. Buratowski. 2004. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13:67-76. - PubMed
-
- Alen, C., N. A. Kent, H. S. Jones, J. O′Sullivan, A. Aranda, and N. J. Proudfoot. 2002. A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol. Cell 10:1441-1452. - PubMed
-
- Beisel, C., A. Imhof, J. Greene, E. Kremmer, and F. Sauer. 2002. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419:857-862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
