Ascites induces modulation of alpha6beta1 integrin and urokinase plasminogen activator receptor expression and associated functions in ovarian carcinoma
- PMID: 15798771
- PMCID: PMC2362012
- DOI: 10.1038/sj.bjc.6602495
Ascites induces modulation of alpha6beta1 integrin and urokinase plasminogen activator receptor expression and associated functions in ovarian carcinoma
Abstract
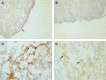
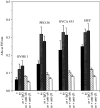
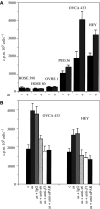
Interactions between cancer cells and the surrounding medium are not fully understood. In this study, we demonstrate that ascites induces selective changes in the expression of integrins and urokinase plasminogen activator/urokinase plasminogen activator receptor (uPA/uPAR) in ovarian cancer cells. We hypothesise that this change of integrin and uPA/uPAR expression triggers signalling pathways responsible for modulating phenotype-dependent functional changes in ovarian cancer cells. Human ovarian surface epithelial (HOSE) cell lines and epithelial ovarian cancer cell lines were treated with ascites for 48 h. Ascites induced upregulation of alpha6 integrin, without any change in the expression of alphav, beta1 and beta4 integrin subunits. Out of the four ovarian cancer cell lines studied, ascites induced enhancement in the expression of uPA/uPAR in the more invasive OVCA 433 and HEY cell lines without any change in the noninvasive OVHS1 and moderately invasive PEO.36 cell lines. On the other hand, no change in the expression of alpha6 integrin or uPAR, in response to ascites, was observed in HOSE cells. In response to ascites, enhancement in proliferation and in adhesion was observed in all four ovarian cancer cell lines studied. In contrast, no significant increase in proliferation or adhesion by ascites was observed in HOSE cells. Ascites-induced expression of uPA/uPAR correlated with the increased invasiveness of HEY and OVCA 433 cell lines but was not seen in OVHS1, PEO.36 and HOSE cell lines. Upregulation of alpha6 integrin and uPA/uPAR correlated with the activation of Ras and downstream Erk pathways. Ascites-induced activation of Ras and downstream Erk can be inhibited by using inhibitory antibodies against alpha6 and beta1 integrin and uPAR, consistent with the inhibition of proliferation, adhesion and invasive functions of ovarian cancer cell lines. Based on these findings, we conclude that ascites can induce selective upregulation of integrin and uPA/uPAR in ovarian cancer cells and these changes may modulate the functions of ovarian carcinomas.
Figures




References
-
- Ahmed N, Niu J, Dorahy DJ, Gu X, Andrews S, Meldrum CJ, Scott RJ, Baker MS, Macreadie IG, Agrez MV (2002a) Direct integrin alphavbeta6-ERK binding: implications for tumour growth. Oncogene 21: 1370–1380 - PubMed
-
- Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok S, Baker MS (2002b) Overexpression of alphavbeta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis 23: 237–244 - PubMed
-
- Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA (2003b) Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol 201: 229–237 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

