Molecular determinants for modulation of persistent sodium current by G-protein betagamma subunits
- PMID: 15800189
- PMCID: PMC6724911
- DOI: 10.1523/JNEUROSCI.0104-05.2005
Molecular determinants for modulation of persistent sodium current by G-protein betagamma subunits
Abstract
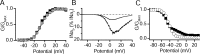
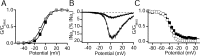
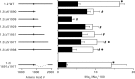
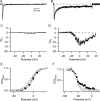
Voltage-gated sodium channels are responsible for the upstroke of the action potential in most excitable cells, and their fast inactivation is essential for controlling electrical signaling. In addition, a noninactivating, persistent component of sodium current, I(NaP), has been implicated in integrative functions of neurons including threshold for firing, neuronal bursting, and signal integration. G-protein betagamma subunits increase I(NaP), but the sodium channel subtypes that conduct I(NaP) and the target site(s) on the sodium channel molecule required for modulation by Gbetagamma are poorly defined. Here, we show that I(NaP) conducted by Na(v)1.1 and Na(v)1.2 channels (Na(v)1.1 > Na(v)1.2) is modulated by Gbetagamma; Na(v)1.4 and Na(v)1.5 channels produce smaller I(NaP) that is not regulated by Gbetagamma. These qualitative differences in modulation by Gbetagamma are determined by the transmembrane body of the sodium channels rather than their cytoplasmic C-terminal domains, which have been implicated previously in modulation by Gbetagamma. However, the C-terminal domains determine the quantitative extent of modulation of Na(v)1.2 channels by Gbetagamma. Studies of chimeric and truncated Na(v)1.2 channels identify molecular determinants that affect modulation of I(NaP) located between amino acid residue 1890 and the C terminus at residue 2005. The last 28 amino acid residues of the C terminus are sufficient to support modulation by Gbetagamma when attached to the proximal C-terminal domain. Our results further define the sodium channel subtypes that generate I(NaP) and identify crucial molecular determinants in the C-terminal domain required for modulation by Gbetagamma when attached to the transmembrane body of a responsive sodium channel.
Figures






Similar articles
-
Origin of the voltage dependence of G-protein regulation of P/Q-type Ca2+ channels.J Neurosci. 2008 Dec 24;28(52):14176-88. doi: 10.1523/JNEUROSCI.1350-08.2008. J Neurosci. 2008. PMID: 19109500 Free PMC article.
-
G protein-gated inhibitory module of N-type (ca(v)2.2) ca2+ channels.Neuron. 2005 Jun 16;46(6):891-904. doi: 10.1016/j.neuron.2005.05.011. Neuron. 2005. PMID: 15953418
-
Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels.J Neurosci. 2009 Feb 18;29(7):2027-42. doi: 10.1523/JNEUROSCI.4531-08.2009. J Neurosci. 2009. PMID: 19228957 Free PMC article.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
-
G protein modulation of CaV2 voltage-gated calcium channels.Channels (Austin). 2010 Nov-Dec;4(6):497-509. doi: 10.4161/chan.4.6.12871. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 21150298 Free PMC article. Review.
Cited by
-
Phenytoin inhibits the persistent sodium current in neocortical neurons by modifying its inactivation properties.PLoS One. 2013;8(1):e55329. doi: 10.1371/journal.pone.0055329. Epub 2013 Jan 29. PLoS One. 2013. PMID: 23383157 Free PMC article.
-
GABA-B Controls Persistent Na+ Current and Coupled Na+-Activated K+ Current.eNeuro. 2017 Jun 23;4(3):ENEURO.0114-17.2017. doi: 10.1523/ENEURO.0114-17.2017. eCollection 2017 May-Jun. eNeuro. 2017. PMID: 28660246 Free PMC article.
-
Persistent sodium currents in SCN1A developmental and degenerative epileptic dyskinetic encephalopathy.Brain Commun. 2021 Oct 7;3(4):fcab235. doi: 10.1093/braincomms/fcab235. eCollection 2021. Brain Commun. 2021. PMID: 34755109 Free PMC article.
-
Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy.J Neurosci. 2007 Oct 10;27(41):11065-74. doi: 10.1523/JNEUROSCI.2162-07.2007. J Neurosci. 2007. PMID: 17928448 Free PMC article.
-
International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy.Pharmacol Rev. 2006 Sep;58(3):281-341. doi: 10.1124/pr.58.3.3. Pharmacol Rev. 2006. PMID: 16968944 Free PMC article. Review.
References
-
- Alonso A, Llinas RR (1989) Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342: 175-177. - PubMed
-
- Brown AM, Schwindt PC, Crill WE (1994) Different voltage dependence of transient and persistent Na+ currents is compatible with modal-gating hypothesis for sodium channels. J Neurophysiol 71: 2562-2565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
