Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses
- PMID: 15800191
- PMCID: PMC6724891
- DOI: 10.1523/JNEUROSCI.4227-04.2005
Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses
Abstract
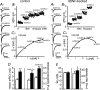
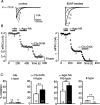
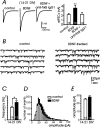
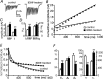
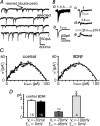
Long-lasting exposures to brain-derived neurotrophic factor (BDNF) accelerate the functional maturation of GABAergic transmission in embryonic hippocampal neurons, but the molecular bases of this phenomenon are still debated. Evidence in favor of a postsynaptic site of action has been accumulated, but most of the data support a presynaptic site effect. A crucial issue is whether the enhancement of evoked IPSCs (eIPSCs) induced by BDNF is attributable to an increase in any of the elementary parameters controlling neurosecretion, namely the probability of release, the number of release sites, the readily releasable pool (RRP), and the quantal size. Here, using peak-scaled variance analysis of miniature IPSCs, multiple probability fluctuation analysis, and cumulative amplitude analysis of action potential-evoked postsynaptic currents, we show that BDNF increases release probability and vesicle replenishment with little or no effect on the quantal size, the number of release sites, the RRP, and the Ca2+ dependence of eIPSCs. BDNF treatment changes markedly the distribution of Ca2+ channels controlling neurotransmitter release. It enhances markedly the contribution of N- and P/Q-type channels, which summed to >100% ("supra-additivity"), and deletes the contribution of R-type channels. BDNF accelerates the switch of presynaptic Ca2+ channel distribution from "segregated" to "nonuniform" distribution. This maturation effect was accompanied by an uncovered increased control of N-type channels on paired-pulse depression, otherwise dominated by P/Q-type channels in untreated neurons. Nevertheless, BDNF preserved the fast recovery from depression associated with N-type channels. These novel presynaptic BDNF actions derive mostly from an enhanced overlapping and better colocalization of N- and P/Q-type channels to vesicle release sites.
Figures
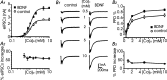

References
-
- Augustine GJ (2001) How does calcium trigger neurotransmitter release? Curr Opin Neurobiol 11: 320-326. - PubMed
-
- Baldelli P, Forni PE, Carbone E (2000) BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci 12: 4017-4032. - PubMed
-
- Baldelli P, Novara M, Carabelli V, Hernández-Guijo JM, Carbone E (2002) BDNF up-regulates evoked GABAergic transmission in developing hippocampal neurons by potentiating presynaptic N- and P/Q-type Ca2+ channel signaling. Eur J Neurosci 16: 2297-2310. - PubMed
-
- Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ (1992) Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature 359: 417-420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
