A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC
- PMID: 15800193
- PMCID: PMC6724907
- DOI: 10.1523/JNEUROSCI.3553-04.2005
A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC
Abstract
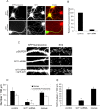
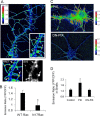
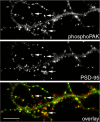
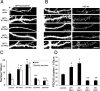
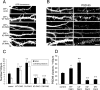
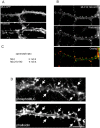
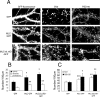
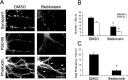
Three of seven recently identified genes mutated in nonsyndromic mental retardation are involved in Rho family signaling. Two of the gene products, alpha-p-21-activated kinase (PAK) interacting exchange factor (alphaPIX) and PAK3, form a complex with the synaptic adaptor protein G-protein-coupled receptor kinase-interacting protein 1 (GIT1). Using an RNA interference approach, we show that GIT1 is critical for spine and synapse formation. We also show that Rac is locally activated in dendritic spines using fluorescence resonance energy transfer. This local activation of Rac is regulated by PIX, a Rac guanine nucleotide exchange factor. PAK1 and PAK3 serve as downstream effectors of Rac in regulating spine and synapse formation. Active PAK promotes the formation of spines and dendritic protrusions, which correlates with an increase in the number of excitatory synapses. These effects are dependent on the kinase activity of PAK, and PAK functions through phosphorylating myosin II regulatory light chain (MLC). Activated MLC causes an increase in dendritic spine and synapse formation, whereas inhibiting myosin ATPase activity results in decreased spine and synapse formation. Finally, both activated PAK and activated MLC can rescue the defects of GIT1 knockdown, suggesting that PAK and MLC are downstream of GIT1 in regulating spine and synapse formation. Our results point to a signaling complex, consisting of GIT1, PIX, Rac, and PAK, that plays an essential role in the regulation of dendritic spine and synapse formation and provides a potential mechanism by which alphaPIX and PAK3 mutations affect cognitive functions in mental retardation.
Figures
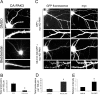
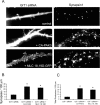
References
-
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA (1998) PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet 20: 25-30. - PubMed
-
- Bienvenu T, des Portes V, McDonell N, Carrie A, Zemni R, Couvert P, Ropers HH, Moraine C, van Bokhoven H, Fryns JP, Allen K, Walsh CA, Boue J, Kahn A, Chelly J, Beldjord C (2000) Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation. Am J Med Genet 93: 294-298. - PubMed
-
- Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB (1998) Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil 19: 839-854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
