Microanatomy of axon/glial signaling during Wallerian degeneration
- PMID: 15800203
- PMCID: PMC6724908
- DOI: 10.1523/JNEUROSCI.3766-04.2005
Microanatomy of axon/glial signaling during Wallerian degeneration
Abstract
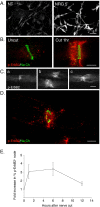
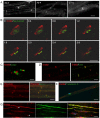
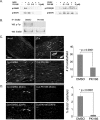
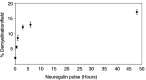
How do myelinated axons signal to the nuclei of cells that enwrap them? The cell bodies of oligodendrocytes and Schwann cells are segregated from axons by multiple layers of bimolecular lipid leaflet and myelin proteins. Conventional signal transduction strategies would seem inadequate to the challenge without special adaptations. Wallerian degeneration provides a model to study axon-to-Schwann cell signaling in the context of nerve injury. We show a hitherto undetected rapid, but transient, activation of the receptor tyrosine kinase erbB2 in myelinating Schwann cells after sciatic nerve axotomy. Deconvolving microscopy using phosphorylation state-specific antibodies shows that erbB2 activation emanates from within the microvilli of Schwann cells, in direct contact with the axons they enwrap. To define the functional role of this transient activation, we used a small molecule antagonist of erbB2 activation (PKI166). The response of myelinating Schwann cells to axotomy is inhibited by PKI166 in vivo. Using neuron/Schwann cell cocultures prepared in compartmentalized cell culture chambers, we show that even transient activation of erbB2 is sufficient to initiate Schwann cell demyelination and that the initiating functions of erbB2 are localized to Schwann cells.
Figures



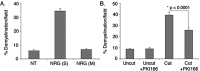
Similar articles
-
A role for Schwann cell-derived neuregulin-1 in remyelination.Nat Neurosci. 2013 Jan;16(1):48-54. doi: 10.1038/nn.3281. Epub 2012 Dec 9. Nat Neurosci. 2013. PMID: 23222914
-
Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration.J Neurosci. 1997 Mar 1;17(5):1642-59. doi: 10.1523/JNEUROSCI.17-05-01642.1997. J Neurosci. 1997. PMID: 9030624 Free PMC article.
-
Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination.J Cell Biol. 2001 Mar 19;152(6):1289-99. doi: 10.1083/jcb.152.6.1289. J Cell Biol. 2001. PMID: 11257128 Free PMC article.
-
Wallerian demyelination: chronicle of a cellular cataclysm.Cell Mol Life Sci. 2017 Nov;74(22):4049-4057. doi: 10.1007/s00018-017-2565-2. Epub 2017 Jun 9. Cell Mol Life Sci. 2017. PMID: 28600652 Free PMC article. Review.
-
Basic Pathological Mechanisms in Peripheral Nerve Diseases.Int J Mol Sci. 2025 Apr 4;26(7):3377. doi: 10.3390/ijms26073377. Int J Mol Sci. 2025. PMID: 40244242 Free PMC article. Review.
Cited by
-
Extracellular Signal-regulated Kinase Activation Is Required for Serine 727 Phosphorylation of STAT3 in Schwann Cells in vitro and in vivo.Korean J Physiol Pharmacol. 2009 Jun;13(3):161-8. doi: 10.4196/kjpp.2009.13.3.161. Epub 2009 Jun 30. Korean J Physiol Pharmacol. 2009. PMID: 19885032 Free PMC article.
-
Axon degeneration: make the Schwann cell great again.Neural Regen Res. 2017 Apr;12(4):518-524. doi: 10.4103/1673-5374.205000. Neural Regen Res. 2017. PMID: 28553320 Free PMC article. Review.
-
Changes in the Coding and Non-coding Transcriptome and DNA Methylome that Define the Schwann Cell Repair Phenotype after Nerve Injury.Cell Rep. 2017 Sep 12;20(11):2719-2734. doi: 10.1016/j.celrep.2017.08.064. Cell Rep. 2017. PMID: 28903050 Free PMC article.
-
Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination.Glia. 2008 Jun;56(8):877-87. doi: 10.1002/glia.20662. Glia. 2008. PMID: 18338795 Free PMC article.
-
Rac1 GTPase controls myelination and demyelination.Bioarchitecture. 2011 May;1(3):110-113. doi: 10.4161/bioa.1.3.16985. Bioarchitecture. 2011. PMID: 21922040 Free PMC article.
References
-
- Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA (1999) NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70S6K, and MEK-MAPK-RSK. Am J Physiol 277: H2026-H2037. - PubMed
-
- Brockes JP, Fields KL, Raff MC (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res 165: 105-118. - PubMed
-
- Buonanno A, Fischbach GD (2001) Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11: 287-296. - PubMed
-
- Burden S, Yarden Y (1997) Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18: 847-855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
