A GFP-based reporter system to monitor nonsense-mediated mRNA decay
- PMID: 15800205
- PMCID: PMC1072805
- DOI: 10.1093/nar/gni052
A GFP-based reporter system to monitor nonsense-mediated mRNA decay
Abstract
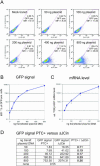
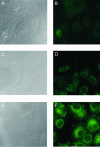
Aberrant mRNAs whose open reading frame (ORF) is truncated by the presence of a premature translation-termination codon (PTC) are recognized and degraded in eukaryotic cells by a process called nonsense-mediated mRNA decay (NMD). Here, we report the development of a reporter system that allows monitoring of NMD in mammalian cells by measuring the fluorescence of green fluorescent protein (GFP). The NMD reporter gene consists of a T-cell receptor-beta minigene construct, in which the GFP-ORF was inserted such that the stop codon of GFP is recognized as PTC. The reporter mRNA is therefore subjected to NMD, resulting in a low steady-state mRNA level, an accordingly low protein level and hence a very low green fluorescence in normal, NMD-competent cells that express this reporter gene. We show that the inactivation of NMD by RNAi-mediated knockdown of the essential NMD factor hUpf1 or hSmg6 increases the NMD reporter mRNA level, resulting in a proportional increase of the green fluorescence that can be detected by flow cytometry, spectrofluorometry and fluorescence microscopy. With these properties, our GFP-based NMD reporter system could be used for large-scale screenings to identify NMD-inhibiting drugs or NMD-deficient mutant cells.
Figures





References
-
- Maquat L.E. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nature Rev. Mol. Cell Biol. 2004;5:89–99. - PubMed
-
- Frischmeyer P.A., Dietz H.C. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 1999;8:1893–1900. - PubMed
-
- Modrek B., Lee C. A genomic view of alternative splicing. Nature Genet. 2002;30:13–19. - PubMed
-
- Mendell J.T., Dietz H.C. When the message goes awry. disease-producing mutations that influence mRNA content and performance. Cell. 2001;107:411–414. - PubMed
