Srs2 and RecQ homologs cooperate in mei-3-mediated homologous recombination repair of Neurospora crassa
- PMID: 15800214
- PMCID: PMC1072801
- DOI: 10.1093/nar/gki326
Srs2 and RecQ homologs cooperate in mei-3-mediated homologous recombination repair of Neurospora crassa
Abstract
Homologous recombination and post-replication repair facilitate restart of stalled or collapsed replication forks. The SRS2 gene of Saccharomyces cerevisiae encodes a 3'-5' DNA helicase that functions both in homologous recombination repair and in post-replication repair. This study identifies and characterizes the SRS2 homolog in Neurospora crassa, which we call mus-50. A knockout mutant of N.crassa, mus-50, is sensitive to several DNA-damaging agents and genetic analyses indicate that it is epistatic with mei-3 (RAD51 homolog), mus-11 (RAD52 homolog), mus-48 (RAD55 homolog) and mus-49 (RAD57 homolog), suggesting a role for mus-50 in homologous recombination repair. However, epistasis evidence has presented that MUS50 does not participate in post-replication repair in N.crassa. Also, the N.crassa mus-25 (RAD54 homolog) mus-50 double mutant is viable, which is in contrast to the lethal phenotype of the equivalent rad54 srs2 mutant in S.cerevisiae. Tetrad analysis revealed that mus-50 in combination with mutations in two RecQ homologs, qde-3 and recQ2, is lethal, and this lethality is suppressed by mutation in mei-3, mus-11 or mus-25. Evidence is also presented for the two independent pathways for recovery from camptothecin-induced replication fork arrest: one pathway is dependent on QDE3 and MUS50 and the other pathway is dependent on MUS25 and RECQ2.
Figures



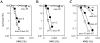


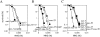

Similar articles
-
Growth defect and mutator phenotypes of RecQ-deficient Neurospora crassa mutants separately result from homologous recombination and nonhomologous end joining during repair of DNA double-strand breaks.Genetics. 2006 Jan;172(1):113-25. doi: 10.1534/genetics.105.041756. Epub 2005 Oct 11. Genetics. 2006. PMID: 16219790 Free PMC article.
-
The Neurospora crassa mus-19 gene is identical to the qde-3 gene, which encodes a RecQ homologue and is involved in recombination repair and postreplication repair.Curr Genet. 2004 Feb;45(1):37-44. doi: 10.1007/s00294-003-0459-3. Epub 2003 Nov 1. Curr Genet. 2004. PMID: 14595518
-
Characterization of the Neurospora crassa mus-25 mutant: the gene encodes a protein which is homologous to the Saccharomyces cerevisiae Rad54 protein.Mol Gen Genet. 2000 Sep;264(1-2):154-63. doi: 10.1007/s004380000303. Mol Gen Genet. 2000. PMID: 11016845
-
Exploring the processes of DNA repair and homologous integration in Neurospora.Mutat Res. 2011 Jul-Oct;728(1-2):1-11. doi: 10.1016/j.mrrev.2011.06.003. Epub 2011 Jul 2. Mutat Res. 2011. PMID: 21757027 Review.
-
Recombination-independent recognition of DNA homology for repeat-induced point mutation.Curr Genet. 2017 Jun;63(3):389-400. doi: 10.1007/s00294-016-0649-4. Epub 2016 Sep 14. Curr Genet. 2017. PMID: 27628707 Free PMC article. Review.
Cited by
-
Neurosprora crassa RAD5 homologue, mus-41, inactivation results in higher sensitivity to mutagens but has little effect on PCNA-ubiquitylation in response to UV-irradiation.Curr Genet. 2007 Sep;52(3-4):125-35. doi: 10.1007/s00294-007-0146-x. Epub 2007 Aug 17. Curr Genet. 2007. PMID: 17703305
-
Rapid genetic mapping in Neurospora crassa.Fungal Genet Biol. 2007 Jun;44(6):455-65. doi: 10.1016/j.fgb.2006.09.002. Epub 2006 Oct 23. Fungal Genet Biol. 2007. PMID: 17056287 Free PMC article.
-
A three-gene cluster in Trichoderma reesei reveals a potential role of dmm2 in DNA repair and cellulase production.Biotechnol Biofuels Bioprod. 2022 Mar 29;15(1):34. doi: 10.1186/s13068-022-02132-y. Biotechnol Biofuels Bioprod. 2022. PMID: 35351200 Free PMC article.
-
A SRS2 homolog from Arabidopsis thaliana disrupts recombinogenic DNA intermediates and facilitates single strand annealing.Nucleic Acids Res. 2009 Nov;37(21):7163-76. doi: 10.1093/nar/gkp753. Nucleic Acids Res. 2009. PMID: 19767619 Free PMC article.
-
The human F-Box DNA helicase FBH1 faces Saccharomyces cerevisiae Srs2 and postreplication repair pathway roles.Mol Cell Biol. 2007 Nov;27(21):7439-50. doi: 10.1128/MCB.00963-07. Epub 2007 Aug 27. Mol Cell Biol. 2007. PMID: 17724085 Free PMC article.
References
-
- Prakash S., Sung P., Prakash L. DNA repair genes and proteins of Saccharomyces cerevisiae. Annu. Rev. Genet. 1993;27:33–70. - PubMed
-
- Friedberg E.C., Bardwell A.J., Bardwell L., Feaver W.J., Kornberg R.D., Svejstrup J.Q., Tomkinson A.E., Wang Z. Nucleotide excision repair in the yeast Saccharomyces cerevisiae: its relationship to specialized mitotic recombination and RNA polymerase II basal transcription. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995;347:63–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

