Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells
- PMID: 15801906
- PMCID: PMC1180729
- DOI: 10.1042/BJ20050337
Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells
Erratum in
- Biochem J. 2005 Sep 15;390(Pt 3):791-2
Abstract
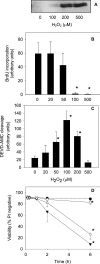
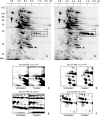
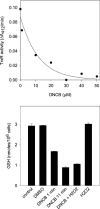
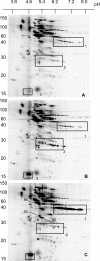
Thiol proteins are important in cellular antioxidant defenses and redox signalling. It is postulated that reactive oxidants cause selective thiol oxidation, but relative sensitivities of different cell proteins and critical targets are not well characterized. We exposed Jurkat cells to H2O2 for 10 min and measured changes in reversibly oxidized proteins by labelling with iodoacetamidofluorescein and two-dimensional electrophoresis. At 200 microM H2O2, which caused activation of the MAP (mitogen-activated protein) kinase ERK (extracellular-signal-regulated kinase), growth arrest and apoptosis, relatively few changes were seen. A total of 28 spots were reversibly oxidized (increased labelling intensity) and 24 decreased. The latter included isoforms of peroxiredoxins 1 and 2, which were irreversibly oxidized. Oxidation of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was striking, and other affected proteins included glutathione S-transferase P1-1, enolase, a regulatory subunit of protein kinase A, annexin VI, the mitotic checkpoint serine/threonine-protein kinase BUB1beta, HSP90beta (heat-shock protein 90beta) and proteosome components. At 20 microM H2O2, changes were fewer, but GAPDH and peroxiredoxin 2 were still modified. Dinitrochlorobenzene treatment, which inhibited cellular thioredoxin reductase and partially depleted GSH, caused reversible oxidation of several proteins, including thioredoxin 1 and peroxiredoxins 1 and 2. Most changes were distinct from those with H2O2, and changes with H2O2 were scarcely enhanced by dinitrochlorobenzene. Relatively few proteins, including deoxycytidine kinase, nucleoside diphosphate kinase and a proteosome activator subunit, responded only to the combined treatment. Thus most of the effects of H2O2 were not linked to thioredoxin oxidation. Our study has identified peroxiredoxin 2 and GAPDH as two of the most oxidant-sensitive cell proteins and has highlighted how readily peroxiredoxins undergo irreversible oxidation.
Figures
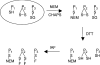


References
-
- Allen R. G., Tresini M. Oxidative stress and gene regulation. Free Radicals Biol. Med. 2000;28:463–499. - PubMed
-
- Burdon R. H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radicals Biol. Med. 1995;18:775–794. - PubMed
-
- Hampton M. B., Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–556. - PubMed
-
- Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. - PubMed
-
- Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000;2:811–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

