Steady-state pharmacokinetics and pharmacodynamics of CHF3381, a novel antineuropathic pain agent, in healthy subjects
- PMID: 15801935
- PMCID: PMC1884805
- DOI: 10.1111/j.1365-2125.2005.02338.x
Steady-state pharmacokinetics and pharmacodynamics of CHF3381, a novel antineuropathic pain agent, in healthy subjects
Abstract
Aims: To evaluate the safety, tolerability, pharmacokinetic and pharmacodynamic profiles of CHF3381, a dual NMDA and MAO-A inhibitor, after multiple oral doses in healthy subjects.
Methods: Forty-eight young males received CHF3381 at doses of 100 mg twice daily, 200 mg twice daily, 400 mg twice daily or placebo for 2 weeks according to a double-blind, randomized, parallel group design. Plasma and urine concentrations of the parent drug and of two major metabolites (CHF3567 and 2-aminoindane) were measured over time. MAO-A activity in plasma was estimated by measuring plasma concentrations of 3,4-dihydroxyphenylglycol. Sustained attention, memory and sedation were assessed throughout the study with standard psychometric tests.
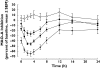
Results: Most of the adverse events were mild in intensity, with dose regimens of 100 mg twice daily and 200 mg twice daily being indistinguishable from placebo. After 400 mg twice daily, the most frequent adverse events were mild dizziness, asthenia and insomnia. At steady-state, 400 mg twice daily slightly increased supine heart rate (+ 9 +/- 2 beats min(-1)) and diastolic blood pressure (+6 +/- 2 mmHg) compared with placebo. There were no dose-dependent or consistent effects of CHF3381 on attention, motor co-ordination or memory, but 400 mg twice daily significantly decreased alertness compared with placebo. Plasma concentrations of CHF3381 peaked at around 3 h and were dose-proportional. The elimination half-life of CHF3381 was estimated to be 4-6 h. At steady-state, significant CHF3381 plasma concentrations were detected at predose with a modest accumulation (1.3-1.5 times), showing that the drug given twice daily is active over the entire 24 h period. Plasma concentrations of CHF3567 and of 2-aminoindane were also proportional to the dose of CHF3381. CHF3381 dose-dependently inhibited MAO-A activity with peak effects at steady-state of 27 +/- 4%, 46 +/- 2% and 65 +/- 5% after 100 mg twice daily, 200 mg twice daily and 400 mg twice daily, respectively. There were no significant effects of CHF3381 on attention (rapid visual information processing), motor co-ordination (body sway) or memory (learning memory task) at any of the doses. At steady-state, there was a significant decrease in alertness (Bond & Lader visual analogue scale) in the 400 mg twice daily group compared with placebo.
Conclusions: A twice daily regimen of CHF3381 appears to be adequate from a pharmacokinetic and pharmacodynamic perspective. Plasma concentrations reached with 400 mg twice daily exceeded those observed in animals receiving pharmacologically active doses in chronic pain models.
Figures


Similar articles
-
Safety, pharmacokinetics, and pharmacodynamics of CHF 3381, a novel N-methyl-D-aspartate antagonist, after single oral doses in healthy subjects.J Clin Pharmacol. 2003 Aug;43(8):901-11. doi: 10.1177/0091270003256137. J Clin Pharmacol. 2003. PMID: 12953347 Clinical Trial.
-
CHF3381, a N-methyl-D-aspartate receptor antagonist and monoamine oxidase-A inhibitor, attenuates secondary hyperalgesia in a human pain model.J Pain. 2006 Aug;7(8):565-74. doi: 10.1016/j.jpain.2006.02.004. J Pain. 2006. PMID: 16885013 Clinical Trial.
-
Mechanistic pharmacokinetic and pharmacodynamic modeling of CHF3381 (2-[(2,3-dihydro-1H-inden-2-yl)amino]acetamide monohydrochloride), a novel N-methyl-D-aspartate antagonist and monoamine oxidase-A inhibitor in healthy subjects.J Pharmacol Exp Ther. 2005 May;313(2):647-57. doi: 10.1124/jpet.104.080457. Epub 2005 Jan 25. J Pharmacol Exp Ther. 2005. PMID: 15671203
-
Indantadol, a novel NMDA antagonist and nonselective MAO inhibitor for the potential treatment of neuropathic pain.IDrugs. 2007 Sep;10(9):636-44. IDrugs. 2007. PMID: 17786847 Review.
-
Pharmacokinetics, Safety, Tolerability, and Pharmacodynamics of Alicapistat, a Selective Inhibitor of Human Calpains 1 and 2 for the Treatment of Alzheimer Disease: An Overview of Phase 1 Studies.Clin Pharmacol Drug Dev. 2019 Apr;8(3):290-303. doi: 10.1002/cpdd.598. Epub 2018 Jul 27. Clin Pharmacol Drug Dev. 2019. PMID: 30052328 Review.
References
-
- McCleane G. Pharmacological management of neuropathic pain. CNS Drugs. 2003;17:1031–43. - PubMed
-
- Woolf CJ American College of Physicians, American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–51. - PubMed
-
- Eide K, Stubhaug A, Oye I, Breivik H. Continuous subcutaneous administration of the N-methyl-D-aspartic acid (NMDA) receptor antagonist ketamine in the treatment of post-herpetic neuralgia. Pain. 1995;61:221–8. - PubMed
-
- Carlsson KC, Hoem NO, Moberg ER, Mathisen LC. Analgesic effect of dextromethorphan in neuropathic pain. Acta Anaesthesiol Scand. 2004;48:328–36. - PubMed
-
- Schreiber S, Getslev V, Weizman A, Pick CG. The antinociceptive effect of moclobemide in mice is mediated by noradrenergic pathways. Neurosci Lett. 1998;253:183–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

