Therapeutic innovation in the European Union: analysis of the drugs approved by the EMEA between 1995 and 2003
- PMID: 15801943
- PMCID: PMC1884813
- DOI: 10.1111/j.1365-2125.2004.02320.x
Therapeutic innovation in the European Union: analysis of the drugs approved by the EMEA between 1995 and 2003
Abstract
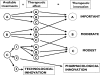
Since January 1995, all European Union applications for marketing approval for medicinal products derived from biotechnology and other drugs considered potentially innovative follow the EMEA centralized procedure. In order to assess the overall degree of therapeutic innovation of these drugs, we considered, for each approved agent, its target, the availability of previous treatments and the extent of its therapeutic effect. The following scores for therapeutic innovation were assigned through a consensus process: 'A' (important), 'B' (moderate) and 'C' (modest). The overall degree of important/moderate therapeutic innovation was 47% of all therapeutic agents (32% important; 15% moderate). Most (80%) of the EMEA-approved therapeutic agents were for serious diseases. The remaining ones were for risk factors (7%) or nonserious diseases (13%).
Figures
Comment in
-
Drug development: more science, more education.Br J Clin Pharmacol. 2005 Apr;59(4):377-8. doi: 10.1111/j.0306-5251.2005.02420.x. Br J Clin Pharmacol. 2005. PMID: 15801930 Free PMC article. No abstract available.
-
On criteria to evaluate the therapeutic innovation of drugs.Br J Clin Pharmacol. 2021 May;87(5):2403-2404. doi: 10.1111/bcp.14650. Epub 2020 Nov 20. Br J Clin Pharmacol. 2021. PMID: 33219565 No abstract available.
Similar articles
-
An update on the first decade of the European centralized procedure: how many innovative drugs?Br J Clin Pharmacol. 2006 Nov;62(5):610-6. doi: 10.1111/j.1365-2125.2006.02700.x. Epub 2006 Jun 23. Br J Clin Pharmacol. 2006. PMID: 16796703 Free PMC article.
-
[European Agency for the Evaluation of Medicinal Products: five years experience].Bull Mem Acad R Med Belg. 2000;155(5-6):254-8; discussion 259-62. Bull Mem Acad R Med Belg. 2000. PMID: 11304960 French.
-
Pharmaceutical biotechnology products approved within the European Union.Eur J Pharm Biopharm. 2003 Jan;55(1):3-10. doi: 10.1016/s0939-6411(02)00165-0. Eur J Pharm Biopharm. 2003. PMID: 12551698 Review.
-
Registries supporting new drug applications.Pharmacoepidemiol Drug Saf. 2017 Dec;26(12):1451-1457. doi: 10.1002/pds.4332. Epub 2017 Oct 6. Pharmacoepidemiol Drug Saf. 2017. PMID: 28983992 Free PMC article.
-
Clinical trials for registration in the European Union: the EMEA 5-year experience in oncology.Crit Rev Oncol Hematol. 2002 May;42(2):123-35. doi: 10.1016/s1040-8428(02)00009-4. Crit Rev Oncol Hematol. 2002. PMID: 12007970 Review.
Cited by
-
Antidepressant-induced Dopamine Receptor Dysregulation: A Valid Animal Model of Manic-Depressive Illness.Curr Neuropharmacol. 2017 Apr;15(3):417-423. doi: 10.2174/1570159X14666160715165648. Curr Neuropharmacol. 2017. PMID: 28503114 Free PMC article. Review.
-
How innovative are new drugs launched in the UK? A retrospective study of new drugs listed in the British National Formulary (BNF) 2001-2012.BMJ Open. 2014 Oct 24;4(10):e006235. doi: 10.1136/bmjopen-2014-006235. BMJ Open. 2014. PMID: 25344485 Free PMC article.
-
No difference in between-country variability in use of newly approved orphan and non- orphan medicinal products--a pilot study.Orphanet J Rare Dis. 2009 Dec 14;4:27. doi: 10.1186/1750-1172-4-27. Orphanet J Rare Dis. 2009. PMID: 20003427 Free PMC article.
-
How innovation can be defined, evaluated and rewarded in health technology assessment.Health Econ Rev. 2022 Jan 3;12(1):1. doi: 10.1186/s13561-021-00342-y. Health Econ Rev. 2022. PMID: 34981266 Free PMC article. Review.
-
Post-approval safety issues with innovative drugs: a European cohort study.Drug Saf. 2013 Nov;36(11):1105-15. doi: 10.1007/s40264-013-0094-y. Drug Saf. 2013. PMID: 24048690
References
-
- Garattini S, Bertele' V. Efficacy, safety and cost of new drugs acting on the central nervous system. Eur J Clin Pharmacol. 2003;59:79–84. - PubMed
-
- Garattini S, Bertele' V. Efficacy, safety and cost of new cardiovascular drugs: a survey. Eur J Clin Pharmacol. 2003;59:701–6. - PubMed
-
- International Society of Drugs Bulletins declaration. 2001. http://www.isdbweb.org/
-
- EMEA 2003 European Public Assessment Reports. http://www.emea.eu.int/index/indexh1.htm.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

