Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors
- PMID: 15802016
- PMCID: PMC1501126
- DOI: 10.1593/neo.04436
Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors
Abstract
The heavy chain of murine ferritin, an iron storage molecule with ferroxidase activity, was developed as a novel endogenous reporter for the detection of gene expression by magnetic resonance imaging (MRI). Expression of both enhanced green fluorescent protein (EGFP) and influenza hemagglutinin (HA)-tagged ferritin were tightly coregulated by tetracycline (TET), using a bidirectional expression vector. C6 cells stably expressing a TET-EGFP-HA-ferritin construct enabled the dynamic detection of TET-regulated gene expression by MRI, followed by independent validation using fluorescence microscopy and histology. MR relaxation rates were significantly elevated both in vitro and in vivo on TET withdrawal, and were consistent with induced expression of ferritin and increase in intracellular iron content. Hence, overexpression of ferritin was sufficient to trigger cellular response, augmenting iron uptake to a degree detectable by MRI. Application of this novel MR reporter gene that generates significant contrast in the absence of exogenously administered substrates opens new possibilities for noninvasive molecular imaging of gene expression by MRI.
Figures
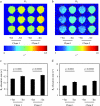
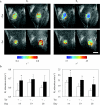
References
-
- Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. - PubMed
-
- Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA, Basilion JP. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6:351–355. - PubMed
-
- Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. - PubMed
-
- Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–2727. - PubMed
-
- Kircher MF, Josephson L, Weissleder R. Ratio imaging of enzyme activity using dual wavelength optical reporters. Mol Imaging. 2002;1:89–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
