Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons
- PMID: 15802470
- PMCID: PMC556262
- DOI: 10.1073/pnas.0501263102
Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons
Abstract
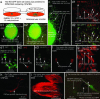
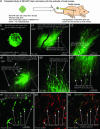
We have recently shown that the expression of nestin, the neural stem cell marker protein, is expressed in bulge-area stem cells of the hair follicle. We used transgenic mice with GFP expression driven by the nestin regulatory element [nestin-driven GFP (ND-GFP)]. The ND-GFP stem cells give rise to the outer-root sheath of the hair follicle as well as an ND-GFP interfollicular vascular network. In this study, we demonstrate that ND-GFP stem cells isolated from the hair-follicle bulge area that are negative for the keratinocyte marker keratin 15 can differentiate into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro. These pluripotent ND-GFP stem cells are positive for the stem cell marker CD34, as well as keratin 15-negative, suggesting their relatively undifferentiated state. The apparent primitive state of the ND-GFP stem cells is compatible with their pluripotency. Furthermore, we show that cells derived from ND-GFP stem cells can differentiate into neurons after transplantation to the subcutis of nude mice. These results suggest that hair-follicle bulge-area ND-GFP stem cells may provide an accessible, autologous source of undifferentiated multipotent stem cells for therapeutic application.
Figures

References
-
- Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T.-T. & Lavker, R. M. (2000) Cell 102, 451–461. - PubMed
-
- Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233–245. - PubMed
-
- Morris, R. J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., Lin, J. S., Sawicki, J. A. & Cotsarelis, G. (2004) Nat. Biotechnol. 22, 411–417. - PubMed
-
- Toma, J. G., Akhavan, M., Fernandes, K. J., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R. & Miller, F. D. (2001) Nat. Cell Biol. 3, 778–784. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

