Functional modulation of human delta opioid receptor by neuropeptide FF
- PMID: 15804369
- PMCID: PMC1079869
- DOI: 10.1186/1471-2202-6-21
Functional modulation of human delta opioid receptor by neuropeptide FF
Abstract
Background: Neuropeptide FF (NPFF) plays a role in physiological pain sensation and opioid analgesia. For example, NPFF potentiates opiate-induced analgesia and the delta opioid receptor antagonist naltrindole inhibits NPFF-induced antinociception. The nature of the interactions between NPFF and opioid receptors seems to be complex and the molecular mechanisms behind the observed physiological effects are not known.
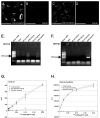
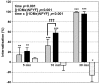
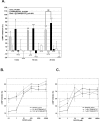
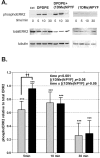
Results: We used a stable Chinese hamster ovary cell line expressing c-MYC-tagged human delta opioid receptor to study the interactions at the molecular level. Our results imply that NPFF can directly modulate the activation of delta opioid receptor in the absence of NPFF receptors. The modulatory effect, though only moderate, was consistently detected with several methods. The agonist-induced receptor trafficking was changed in the presence of (1DMe)NPYF, a stable NPFF-analogue. (1DMe)NPYF enhanced the receptor activation and recovery; opioid antagonists inhibited the effects, indicating that they were delta opioid receptor-mediated. The binding experiments with a novel ligand, Terbium-labeled deltorphin I, showed that (1DMe)NPYF modulated the binding of delta opioid receptor ligands. The levels of phosphorylated mitogen-activated protein kinase and intracellular cAMP were studied to clarify the effects of NPFF on the opioid signaling mechanisms. Application of (1DMe)NPYF together with a delta opioid receptor agonist enhanced the signaling via both pathways studied. Concomitantly to the receptor trafficking, the time-course of the activation of the signaling was altered.
Conclusion: In addition to working via indirect mechanisms on the opioid systems, NPFF may exert a direct modulatory effect on the delta opioid receptor. NPFF may be a multi-functional neuropeptide that regulates several neuronal systems depending on the site of action.
Figures

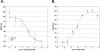
Similar articles
-
Role of the delta-opioid receptor in (1DMe)NPYF mediated antinociception.Peptides. 2001 Jan;22(1):33-8. doi: 10.1016/s0196-9781(00)00353-3. Peptides. 2001. PMID: 11179595
-
Anoretic effects of neuropeptide FF are mediated via central mu and kappa subtypes of opioid receptors and receptor ligands.Gen Comp Endocrinol. 2008 Nov-Dec;159(2-3):125-9. doi: 10.1016/j.ygcen.2008.09.001. Epub 2008 Sep 14. Gen Comp Endocrinol. 2008. PMID: 18823989
-
Functional characterization of a human receptor for neuropeptide FF and related peptides.Br J Pharmacol. 2001 May;133(1):138-44. doi: 10.1038/sj.bjp.0704038. Br J Pharmacol. 2001. PMID: 11325803 Free PMC article.
-
Modulatory role of neuropeptide FF system in nociception and opiate analgesia.Neuropeptides. 2008 Feb;42(1):1-18. doi: 10.1016/j.npep.2007.06.004. Epub 2007 Sep 12. Neuropeptides. 2008. PMID: 17854890 Review.
-
Neuropeptide FF and modulation of pain.Brain Res. 1999 Nov 27;848(1-2):191-6. doi: 10.1016/s0006-8993(99)02044-2. Brain Res. 1999. PMID: 10612711 Review.
Cited by
-
The hypothalamic neuropeptide FF network is impaired in hypertensive patients.Brain Behav. 2014 Jul;4(4):453-67. doi: 10.1002/brb3.229. Epub 2014 Apr 10. Brain Behav. 2014. PMID: 25161813 Free PMC article.
-
Involvement of Mammalian RF-Amide Peptides and Their Receptors in the Modulation of Nociception in Rodents.Front Endocrinol (Lausanne). 2014 Oct 2;5:158. doi: 10.3389/fendo.2014.00158. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25324831 Free PMC article. Review.
-
Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells.Neurochem Res. 2008 Oct;33(10):2028-34. doi: 10.1007/s11064-008-9678-9. Epub 2008 Mar 26. Neurochem Res. 2008. PMID: 18365312
References
-
- Vilim FS, Aarnisalo AA, Nieminen M-L, Lintunen M, Karlstedt K, Kontinen VK, Kalso E, States B, Panula P, Ziff E. Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli. Mol Pharmacol. 1999;55:804–811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

