Unraveling protein-protein interactions in living cells with fluorescence fluctuation brightness analysis
- PMID: 15805168
- PMCID: PMC1305664
- DOI: 10.1529/biophysj.105.059170
Unraveling protein-protein interactions in living cells with fluorescence fluctuation brightness analysis
Abstract
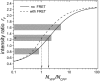
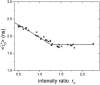
Fluorescence correlation spectroscopy is a potentially powerful tool for measuring protein-protein interactions directly in single living cells. We previously reported on the detection of homodimer formation in cells using molecular brightness analysis. Here, we extend the technique to detect binding between different proteins. Proteins are labeled with the fluorescent markers YFP and CFP. We first determine the coexpression ratio of both proteins by measuring the intensity ratio with a dual-color setup. The effect of fluorescence resonance energy transfer on the intensity ratio is explicitly taken into account. The brightness of cells coexpressing both proteins is measured in a single-color setup. Selecting the laser wavelength of the two-photon light source allows us to either coexcite both proteins or to selectively excite YFP-labeled proteins. This approach enables us to distinguish between homodimer and heterodimer formation. We first present the theory and then demonstrate experimental feasibility using the ligand binding domains of retinoic acid receptor (RARLBD) and of retinoid X receptor (RXRLBD). Both proteins form heterodimers, and RXRLBD also forms homodimers in the presence of its agonist. We explore binding between these proteins in the presence and absence of RXR agonist. Our results demonstrate that brightness analysis offers a quantitative method for determining protein interactions in cells.
Figures



References
-
- Berland, K. M. 1995. Two-Photon Fluctuation Correlation Spectroscopy: Method and Applications to Protein Aggregation and Intracellular Diffusion. University of Illinois at Urbana-Champaign, Urbana, IL.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

