Chiral differentiation of DNA adducts formed by enantiomeric analogues of antitumor cisplatin is sequence-dependent
- PMID: 15805172
- PMCID: PMC1305646
- DOI: 10.1529/biophysj.104.054650
Chiral differentiation of DNA adducts formed by enantiomeric analogues of antitumor cisplatin is sequence-dependent
Abstract
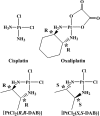
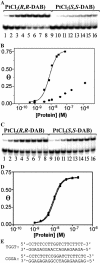
1,2-GG intrastrand cross-links formed in DNA by the enantiomeric complexes [PtCl(2)(R,R-2,3-diaminobutane (DAB))] and [PtCl(2)(S,S-DAB)] were studied by biophysical methods. Molecular modeling revealed that structure of the cross-links formed at the TGGT sequence was affected by repulsion between the 5'-directed methyl group of the DAB ligand and the methyl group of the 5'-thymine of the TGGT fragment. Molecular dynamics simulations of the solvated platinated duplexes and our recent structural data indicated that the adduct of [PtCl(2)(R,R-DAB)] alleviated this repulsion by unwinding the TpG step, whereas the adduct of [PtCl(2)(S,S-DAB)] avoided the unfavorable methyl-methyl interaction by decreasing the kink angle. Electrophoretic retardation measurements on DNA duplexes containing 1,2-GG intrastrand cross-links of Pt(R,R-DAB)(2+) or Pt(S,S-DAB)(2+) at a CGGA site showed that in this sequence both enantiomers distorted the double helix to the identical extent similar to that found previously for the same sequence containing the cross-links of the parent antitumor cis-Pt(NH(3))(2)(2+) (cisplatin). In addition, the adducts showed similar affinities toward the high-mobility-group box 1 proteins. Hence, whereas the structural perturbation induced in DNA by 1,2-GG intrastrand cross-links of cisplatin does not depend largely on the bases flanking the cross-links, the perturbation related to GG cross-linking by bulkier platinum diamine derivatives does.
Figures
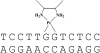


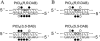

Similar articles
-
DNA interactions of antitumor cisplatin analogs containing enantiomeric amine ligands.Biophys J. 2000 Apr;78(4):2008-21. doi: 10.1016/S0006-3495(00)76748-8. Biophys J. 2000. PMID: 10733979 Free PMC article.
-
The thermodynamics of translesion DNA synthesis past major adducts of enantiomeric analogues of antitumor cisplatin.Chem Asian J. 2012 May;7(5):1026-31. doi: 10.1002/asia.201100886. Epub 2012 Feb 28. Chem Asian J. 2012. PMID: 22374916
-
DNA binding by antitumor trans-[PtCl2(NH3)(thiazole)]. Protein recognition and nucleotide excision repair of monofunctional adducts.Biochemistry. 2003 Jan 28;42(3):792-800. doi: 10.1021/bi026614t. Biochemistry. 2003. PMID: 12534292
-
Trans-diammineplatinum(II): what makes it different from cis-DDP? Coordination chemistry of a neglected relative of cisplatin and its interaction with nucleic acids.Met Ions Biol Syst. 1996;33:105-41. Met Ions Biol Syst. 1996. PMID: 8742842 Review.
-
Interstrand cross-links of cisplatin induce striking distortions in DNA.J Inorg Biochem. 1999 Oct;77(1-2):23-9. doi: 10.1016/s0162-0134(99)00148-8. J Inorg Biochem. 1999. PMID: 10626349 Review.
Cited by
-
Impacts of amino acid-linked platinum(II) complexes on DNA structure.J Biol Inorg Chem. 2025 Feb;30(1):87-101. doi: 10.1007/s00775-025-02097-x. Epub 2025 Jan 24. J Biol Inorg Chem. 2025. PMID: 39853368 Free PMC article.
-
Multimodal optical sensing and analyte specificity using single-walled carbon nanotubes.Nat Nanotechnol. 2009 Feb;4(2):114-20. doi: 10.1038/nnano.2008.369. Epub 2008 Dec 14. Nat Nanotechnol. 2009. PMID: 19197314
-
Conformation of DNA GG intrastrand cross-link of antitumor oxaliplatin and its enantiomeric analog.Biophys J. 2007 Dec 1;93(11):3950-62. doi: 10.1529/biophysj.107.116996. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704160 Free PMC article.
References
-
- Ano, S. O., Z. Kuklenyik, and L. G. Marzilli. 1999. Structure and dynamics of Pt anticancer drug adducts from nucleotides to oligonucleotides as revealed by NMR methods. In Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. B. Lippert, editor. Verlag Helvetica Chimica Acta, Zürich. 247–291.
-
- Bailly, C., and M. J. Waring. 1997. Diethylpyrocarbonate and osmium tetroxide as probes for drug-induced changes in DNA conformation in vitro. In Drug-DNA Interaction Protocols. K. R. Fox, editor. Humana Press, Totowa, NJ. 51–79. - PubMed
-
- Bellon, S. F., J. H. Coleman, and S. J. Lippard. 1991. DNA unwinding produced by site-specific intrastrand cross-links of the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 30:8026–8035. - PubMed
-
- Bellon, S. F., and S. J. Lippard. 1990. Bending studies of DNA site-specifically modified by cisplatin, trans-diamminedichloroplatinum(II) and cis-[Pt(NH3)2(N3-Cytosine)Cl]+. Biophys. Chem. 35:179–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

