Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers
- PMID: 15805181
- PMCID: PMC2718449
- DOI: 10.1164/rccm.200412-1687OC
Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers
Abstract
Rationale: Airway infection with Haemophilus influenzae causes airway inflammation, and isolation of new strains of this bacteria is associated with increased risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD).
Objective: To determine whether strains of H. influenzae associated with exacerbations cause more inflammation than strains that colonize the airways of patients with COPD.
Methods: Exacerbation strains of H. influenzae were isolated from patients during exacerbation of clinical symptoms with subsequent development of a homologous serum antibody response and were compared with colonization strains that were not associated with symptom worsening or an antibody response. Bacterial strains were compared using an in vivo mouse model of airway infection and in vitro cell culture model of bacterial adherence and defense gene and signaling pathway activation in primary human airway epithelial cells.
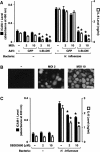
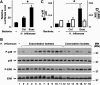
Results: H. influenzae associated with exacerbations caused more airway neutrophil recruitment compared with colonization strains in the mouse model of airway bacterial infection. Furthermore, exacerbation strains adhered to epithelial cells in significantly higher numbers and induced more interleukin-8 release after interaction with airway epithelial cells. This effect was likely mediated by increased activation of the nuclear factor-kappaB and p38 mitogen-activated protein kinase signaling pathways.
Conclusions: The results indicate that H. influenzae strains isolated from patients during COPD exacerbations often induce more airway inflammation and likely have differences in virulence compared with colonizing strains. These findings support the concept that bacteria infecting the airway during COPD exacerbations mediate increased airway inflammation and contribute to decreased airway function.
Figures
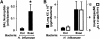
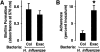
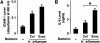
References
-
- Burrows B, Earle RH. Course and prognosis of chronic obstructive lung disease: a prospective study of 200 patients. N Engl J Med 1969;280:397–404. - PubMed
-
- Connors AFJ, Dawson NV, Thomas C, Harrell FEJ, Desbiens N, Fulkerson WJ, Kussin P, Bellamy P, Goldman L, Knaus WA. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959–967. - PubMed
-
- Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

