Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9
- PMID: 15805190
- PMCID: PMC556275
- DOI: 10.1073/pnas.0501652102
Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9
Abstract
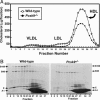
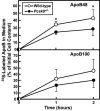
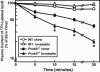
PCSK9 encodes proprotein convertase subtilisin/kexin type 9a (PCSK9), a member of the proteinase K subfamily of subtilases. Missense mutations in PCSK9 cause an autosomal dominant form of hypercholesterolemia in humans, likely due to a gain-of-function mechanism because overexpression of either WT or mutant PCSK9 reduces hepatic LDL receptor protein (LDLR) in mice. Here, we show that livers of knockout mice lacking PCSK9 manifest increased LDLR protein but not mRNA. Increased LDLR protein led to increased clearance of circulating lipoproteins and decreased plasma cholesterol levels (46 mg/dl in Pcsk9(-/-) mice versus 96 mg/dl in WT mice). Statins, a class of drugs that inhibit cholesterol synthesis, increase expression of sterol regulatory element-binding protein-2 (SREBP-2), a transcription factor that activates both the Ldlr and Pcsk9 genes. Statin administration to Pcsk9(-/-) mice produced an exaggerated increase in LDLRs in liver and enhanced LDL clearance from plasma. These data demonstrate that PCSK9 regulates the amount of LDLR protein in liver and suggest that inhibitors of PCSK9 may act synergistically with statins to enhance LDLRs and reduce plasma cholesterol.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

