A checkpoint control linking meiotic S phase and recombination initiation in fission yeast
- PMID: 15805194
- PMCID: PMC556284
- DOI: 10.1073/pnas.0407236102
A checkpoint control linking meiotic S phase and recombination initiation in fission yeast
Abstract
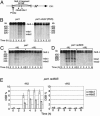
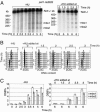
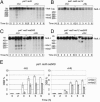
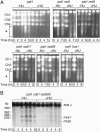
During meiosis, high levels of recombination initiated by DNA double-strand breaks (DSBs) occur only after DNA replication. However, how DSB formation is coupled to DNA replication is unknown. We examined several DNA replication proteins for a role in this coupling in Schizosaccharomyces pombe, and we show that ribonucleotide reductase, the rate-limiting enzyme of deoxyribonucleotide synthesis and the target of the DNA synthesis inhibitor hydroxyurea (HU) is indirectly required for DSB formation linked to DNA replication. However, in cells in which the function of the DNA-replication-checkpoint proteins Rad1p, Rad3p, Rad9p, Rad17p, Rad26p, Hus1p, or Cds1p was compromised, DSB formation occurred at similar frequencies in the absence or presence of HU. The DSBs in the HU-treated mutant cells occurred at normal sites and were associated with recombination. In addition, Cdc2p is apparently not involved in this process. We propose that the sequence of meiotic S phase and initiation of recombination is coordinated by DNA-replication-checkpoint proteins.
Figures

Similar articles
-
Regulation of premeiotic S phase and recombination-related double-strand DNA breaks during meiosis in fission yeast.Nat Genet. 2001 Jul;28(3):290-3. doi: 10.1038/90142. Nat Genet. 2001. PMID: 11431703
-
Rad3-Cds1 mediates coupling of initiation of meiotic recombination with DNA replication. Mei4-dependent transcription as a potential target of meiotic checkpoint.J Biol Chem. 2006 Jan 20;281(3):1338-44. doi: 10.1074/jbc.M505767200. Epub 2005 Nov 14. J Biol Chem. 2006. PMID: 16286472
-
Physical interactions among human checkpoint control proteins HUS1p, RAD1p, and RAD9p, and implications for the regulation of cell cycle progression.Genomics. 2000 Apr 1;65(1):24-33. doi: 10.1006/geno.2000.6142. Genomics. 2000. PMID: 10777662
-
[The role of pre-meiotic S phase].Tanpakushitsu Kakusan Koso. 2002 Jan;47(1):45-50. Tanpakushitsu Kakusan Koso. 2002. PMID: 11808194 Review. Japanese. No abstract available.
-
Mcml0 and DNA replication in fission yeast.SEB Exp Biol Ser. 2008;59:45-69. SEB Exp Biol Ser. 2008. PMID: 18368917 Review. No abstract available.
Cited by
-
Roles of CDK and DDK in Genome Duplication and Maintenance: Meiotic Singularities.Genes (Basel). 2017 Mar 20;8(3):105. doi: 10.3390/genes8030105. Genes (Basel). 2017. PMID: 28335524 Free PMC article. Review.
-
Meiotic Recombination in Schizosaccharomyces pombe: A Paradigm for Genetic and Molecular Analysis.Genome Dyn Stab. 2008 Jan 1;3:195. doi: 10.1007/7050_2007_025. Genome Dyn Stab. 2008. PMID: 20157622 Free PMC article.
-
The histone variant H2A.Z promotes initiation of meiotic recombination in fission yeast.Nucleic Acids Res. 2018 Jan 25;46(2):609-620. doi: 10.1093/nar/gkx1110. Nucleic Acids Res. 2018. PMID: 29145618 Free PMC article.
-
Arnicolide D Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells through PI3K/Akt/mTOR Pathway.Anticancer Agents Med Chem. 2024;24(17):1288-1294. doi: 10.2174/0118715206289595240105082138. Anticancer Agents Med Chem. 2024. PMID: 38967079
-
Cyclins and CDKs in the regulation of meiosis-specific events.Front Cell Dev Biol. 2022 Nov 29;10:1069064. doi: 10.3389/fcell.2022.1069064. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36523509 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

