Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage
- PMID: 15805310
- PMCID: PMC1074328
- DOI: 10.1101/lm.86605
Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage
Abstract
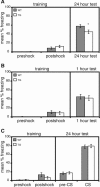
Deletions, translocations, or point mutations in the CREB-binding protein (CBP) gene have been associated with Rubinstein-Taybi Syndrome; a human developmental disorder characterized by retarded growth and reduced mental function. To examine the role of CBP in memory, transgenic mice were generated in which the CaMKII alpha promoter drives expression of an inhibitory truncated CBP protein in forebrain neurons. Examination of hippocampal long-term potentiation (LTP), a form of synaptic plasticity thought to underlie memory storage, revealed significantly reduced late-phase LTP induced by dopamine-regulated potentiation in hippocampal slices from CBP transgenic mice. However, four-train induced late-phase LTP is normal. Behaviorally, CBP transgenic mice exhibited memory deficits in spatial learning in the Morris water maze and deficits in long-term memory for contextual fear conditioning, two hippocampus-dependent tasks. Together, these results demonstrate that CBP is involved in specific forms of hippocampal synaptic plasticity and hippocampus-dependent long-term memory formation.
Figures





Comment in
-
What's right with my mouse model? New insights into the molecular and cellular basis of cognition from mouse models of Rubinstein-Taybi Syndrome.Learn Mem. 2005 Mar-Apr;12(2):80-3. doi: 10.1101/lm.93505. Learn Mem. 2005. PMID: 15805305 Review. No abstract available.
References
-
- Abel, T., Nguyen, P.V., Barad, M., Deuel, T.A., Kandel, E.R., and Bourtchouladze, R. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615-626. - PubMed
-
- Alarcon, J.M., Malleret, G., Touzani, K., Vronskaya, S., Ishii, S., Kandel, E.R., and Barco, A. 2004. Chromatin acetylation, memory, and LTP are impaired in CBP(+/-) mice: A model for the cognitive deficit in Rubinstein-Taybi Syndrome and its amelioration. Neuron 42: 947-959. - PubMed
-
- Bliss, T.V. and Richter-Levin, G. 1993. Spatial learning and the saturation of long-term potentiation. Hippocampus 3: 123-125. - PubMed
-
- Bolstad, B.M., Irizarry, R.A., Astrand, M., and Speed, T.P. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185-193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
