Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT
- PMID: 15805468
- PMCID: PMC1074314
- DOI: 10.1101/gad.1289405
Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT
Abstract
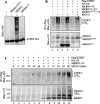
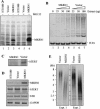
Telomere homeostasis is regulated by telomerase and a collection of associated proteins. Telomerase is, in turn, regulated by post-translational modifications of the rate-limiting catalytic subunit hTERT. Here we show that disruption of Hsp90 by geldanamycin promotes efficient ubiquitination and proteasome-mediated degradation of hTERT. Furthermore, we have used the yeast two-hybrid method to identify a novel RING finger gene (MKRN1) encoding an E3 ligase that mediates ubiquitination of hTERT. Overexpression of MKRN1 in telomerase-positive cells promotes the degradation of hTERT and decreases telomerase activity and subsequently telomere length. Our data suggest that MKRN1 plays an important role in modulating telomere length homeostasis through a dynamic balance involving hTERT protein stability.
Figures

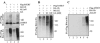

References
-
- Blackburn E.H. 1992. Telomerases. Annu. Rev. Biochem. 61: 113–129. - PubMed
-
- Blasco M.A., Lee, H.W., Hande, M.P., Samper, E., Lansdorp, P.M., DePinho, R.A., and Greider, C.W. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34. - PubMed
-
- Connell P., Ballinger, C.A., Jiang, J., Wu, Y., Thompson, L.J., Hohfeld, J., and Patterson, C. 2001. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3: 93–96. - PubMed
-
- Forsythe H.L., Jarvis, J.L., Turner, J.W., Elmore, L.W., and Holt, S.E. 2001. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J. Biol. Chem. 276: 15571–15574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
