Virus induction of heat shock protein 70 reflects a general response to protein accumulation in the plant cytosol
- PMID: 15805473
- PMCID: PMC1104204
- DOI: 10.1104/pp.104.058958
Virus induction of heat shock protein 70 reflects a general response to protein accumulation in the plant cytosol
Abstract
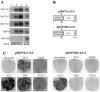
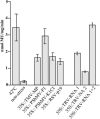
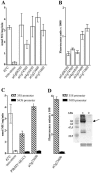
Different cytoplasmically replicating RNA viruses were shown to induce a specific subset of heat-inducible heat shock protein 70 (HSP70) genes in Arabidopsis (Arabidopsis thaliana). To identify the inducing principle, a promoterreporter system was developed for the facile analysis of differentially responding Arabidopsis HSP70 genes, by infiltration into Nicotiana benthamiana leaves. Through transient expression of individual viral cistrons or through deletion analysis of a viral replicon, we were unable to identify a unique inducer of HSP70. However, there was a positive correlation between the translatability of the test construct and the differential induction of HSP70. Since these data implied a lack of specificity in the induction process, we also expressed a random series of cytosolically targeted Arabidopsis genes and showed that these also differentially induced HSP70. Through a comparison of different promoterreporter constructs and through measurements of the steady-state levels of the individual proteins, it appeared that the HSP70 response reflected the ability of the cytosol to sense individual properties of particular proteins when expressed at high levels. This phenomenon is reminiscent of the unfolded protein response observed when the induced accumulation of proteins in the endoplasmic reticulum also induces a specific suite of chaperones.
Figures
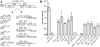
Similar articles
-
A subclass of HSP70s regulate development and abiotic stress responses in Arabidopsis thaliana.J Plant Res. 2017 Mar;130(2):349-363. doi: 10.1007/s10265-016-0900-6. Epub 2016 Dec 22. J Plant Res. 2017. PMID: 28004282
-
The role of Arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance.Plant J. 2010 May 1;62(4):539-48. doi: 10.1111/j.1365-313X.2010.04173.x. Plant J. 2010. PMID: 20536787
-
Evolutionary Conservation and Emerging Functional Diversity of the Cytosolic Hsp70:J Protein Chaperone Network of Arabidopsis thaliana.G3 (Bethesda). 2017 Jun 7;7(6):1941-1954. doi: 10.1534/g3.117.042291. G3 (Bethesda). 2017. PMID: 28450372 Free PMC article.
-
AtHsp70-15-deficient Arabidopsis plants are characterized by reduced growth, a constitutive cytosolic protein response and enhanced resistance to TuMV.Plant J. 2011 Jun;66(6):983-95. doi: 10.1111/j.1365-313X.2011.04558.x. Epub 2011 Apr 28. Plant J. 2011. PMID: 21418353
-
Heat stress-dependent DNA binding of Arabidopsis heat shock transcription factor HSF1 to heat shock gene promoters in Arabidopsis suspension culture cells in vivo.Biol Chem. 2003 Jun;384(6):959-63. doi: 10.1515/BC.2003.108. Biol Chem. 2003. PMID: 12887064
Cited by
-
Sharka: the past, the present and the future.Viruses. 2012 Nov 7;4(11):2853-901. doi: 10.3390/v4112853. Viruses. 2012. PMID: 23202508 Free PMC article. Review.
-
Genome-wide identification and expression analysis of the Hsp20, Hsp70 and Hsp90 gene family in Dendrobium officinale.Front Plant Sci. 2022 Aug 10;13:979801. doi: 10.3389/fpls.2022.979801. eCollection 2022. Front Plant Sci. 2022. PMID: 36035705 Free PMC article.
-
Insights Into Natural Genetic Resistance to Rice Yellow Mottle Virus and Implications on Breeding for Durable Resistance.Front Plant Sci. 2021 Jun 29;12:671355. doi: 10.3389/fpls.2021.671355. eCollection 2021. Front Plant Sci. 2021. PMID: 34267770 Free PMC article. Review.
-
The Role of Plant Latex in Virus Biology.Viruses. 2023 Dec 27;16(1):47. doi: 10.3390/v16010047. Viruses. 2023. PMID: 38257746 Free PMC article. Review.
-
The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis.Plant Cell. 2009 Feb;21(2):642-54. doi: 10.1105/tpc.108.062596. Epub 2009 Feb 24. Plant Cell. 2009. PMID: 19244141 Free PMC article.
References
-
- Aranda MA, Escaler M, Thomas CL, Maule AJ (1999) A heat shock transcription factor in pea is differentially controlled by heat and virus replication. Plant J 20: 153–161 - PubMed
-
- Aranda MA, Maule AJ (1998) Virus-induced host gene shut-off in animals and plants. Virology 243: 261–267 - PubMed
-
- Bendahmane A, Querci M, Kanyuka K, Baulcombe DC (2000) Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: application to the Rx2 locus in potato. Plant J 21: 73–81 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

