Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility
- PMID: 15805509
- PMCID: PMC1070399
- DOI: 10.1128/JB.187.8.2628-2637.2005
Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility
Abstract
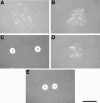
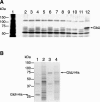
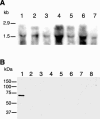
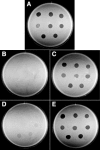
Cells of Flavobacterium johnsoniae glide rapidly over surfaces by an unknown mechanism. Eight genes required for gliding motility have been described. Complementation of the nonmotile mutant UW102-48 identified another gene, gldJ, that is required for gliding. gldJ mutants formed nonspreading colonies, and individual cells were completely nonmotile. Like previously described nonmotile mutants, gldJ mutants were deficient in chitin utilization and were resistant to bacteriophages that infect wild-type cells. Cell fractionation and labeling studies with [(3)H]palmitate indicated that GldJ is a lipoprotein. Mutations in gldA, gldB, gldD, gldF, gldG, gldH, or gldI resulted in normal levels of gldJ transcript but decreased levels of GldJ protein. Expression of truncated GldJ protein in wild-type cells resulted in a severe motility defect. GldJ was found in regular bands that suggest the presence of a helical structure within the cell envelope.
Figures




References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Bayley, D. P., E. R. Rocha, and C. J. Smith. 2000. Analysis of cepA and other Bacteroides fragilis genes reveals a unique promoter structure. FEMS Microbiol. Lett. 193:149-154. - PubMed
-
- Beatson, P. J., and K. C. Marshall. 1994. A proposed helical mechanism for gliding motility in three gliding bacteria (order Cytophagales). Can. J. Microbiol. 40:173-183.
-
- Bhaya, D., N. R. Bianco, D. Bryant, and A. R. Grossman. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 37:941-951. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

