Identification of cyclic AMP-regulated genes in Mycobacterium tuberculosis complex bacteria under low-oxygen conditions
- PMID: 15805514
- PMCID: PMC1070381
- DOI: 10.1128/JB.187.8.2681-2692.2005
Identification of cyclic AMP-regulated genes in Mycobacterium tuberculosis complex bacteria under low-oxygen conditions
Abstract
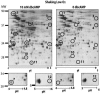
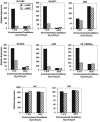
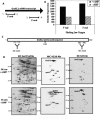
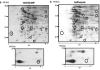
Mycobacterium tuberculosis is the etiological agent of tuberculosis (TB), which kills approximately 2 million people a year despite current treatment options. A greater understanding of the biology of this bacterium is needed to better combat TB disease. The M. tuberculosis genome encodes as many as 15 adenylate cyclases, suggesting that cyclic AMP (cAMP) has an important, yet overlooked, role in mycobacteria. This study examined the effect of exogenous cAMP on protein expression in Mycobacterium bovis BCG grown under hypoxic versus ambient conditions. Both shaking and shallow standing cultures were examined for each atmospheric condition. Different cAMP-dependent changes in protein expression were observed in each condition by two-dimensional gel electrophoresis. Shaking low-oxygen cultures produced the most changes (12), while standing ambient conditions showed the fewest (2). Five upregulated proteins, Rv1265, Rv2971, GroEL2, PE_PGRS6a, and malate dehydrogenase, were identified from BCG by mass spectrometry and were shown to also be regulated by cAMP at the mRNA level in both M. tuberculosis H37Rv and BCG. To our knowledge, these data provide the first direct evidence for cAMP-mediated gene regulation in TB complex mycobacteria.
Figures

Similar articles
-
Rv1675c (cmr) regulates intramacrophage and cyclic AMP-induced gene expression in Mycobacterium tuberculosis-complex mycobacteria.Mol Microbiol. 2009 Jan;71(2):434-48. doi: 10.1111/j.1365-2958.2008.06541.x. Epub 2008 Nov 14. Mol Microbiol. 2009. PMID: 19040643 Free PMC article.
-
Cyclic AMP Signaling in Mycobacteria.Microbiol Spectr. 2014 Apr;2(2). doi: 10.1128/microbiolspec.MGM2-0011-2013. Microbiol Spectr. 2014. PMID: 26105822 Review.
-
Identification of a Mycobacterium tuberculosis putative classical nitroreductase gene whose expression is coregulated with that of the acr aene within macrophages, in standing versus shaking cultures, and under low oxygen conditions.Infect Immun. 2002 Mar;70(3):1518-29. doi: 10.1128/IAI.70.3.1518-1529.2002. Infect Immun. 2002. PMID: 11854240 Free PMC article.
-
Identification and characterization of mycobacterial proteins differentially expressed under standing and shaking culture conditions, including Rv2623 from a novel class of putative ATP-binding proteins.Infect Immun. 2001 Sep;69(9):5777-85. doi: 10.1128/IAI.69.9.5777-5785.2001. Infect Immun. 2001. PMID: 11500455 Free PMC article.
-
Cyclic AMP signalling in mycobacteria: redirecting the conversation with a common currency.Cell Microbiol. 2011 Mar;13(3):349-58. doi: 10.1111/j.1462-5822.2010.01562.x. Epub 2010 Dec 28. Cell Microbiol. 2011. PMID: 21199259 Free PMC article. Review.
Cited by
-
Genome-wide screening of pathogenicity islands in Mycobacterium tuberculosis based on the genomic barcode visualization.Mol Biol Rep. 2014 Sep;41(9):5883-9. doi: 10.1007/s11033-014-3463-4. Epub 2014 Aug 10. Mol Biol Rep. 2014. PMID: 25108673
-
Role of intragenic binding of cAMP responsive protein (CRP) in regulation of the succinate dehydrogenase genes Rv0249c-Rv0247c in TB complex mycobacteria.Nucleic Acids Res. 2015 Jun 23;43(11):5377-93. doi: 10.1093/nar/gkv420. Epub 2015 May 4. Nucleic Acids Res. 2015. PMID: 25940627 Free PMC article.
-
Convergence of two global regulators to coordinate expression of essential virulence determinants of Mycobacterium tuberculosis.Elife. 2022 Nov 9;11:e80965. doi: 10.7554/eLife.80965. Elife. 2022. PMID: 36350294 Free PMC article.
-
Pharmacological and genetic activation of cAMP synthesis disrupts cholesterol utilization in Mycobacterium tuberculosis.PLoS Pathog. 2022 Feb 8;18(2):e1009862. doi: 10.1371/journal.ppat.1009862. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35134095 Free PMC article.
-
Defining Discriminatory Antibody Fingerprints in Active and Latent Tuberculosis.Front Immunol. 2022 Apr 20;13:856906. doi: 10.3389/fimmu.2022.856906. eCollection 2022. Front Immunol. 2022. PMID: 35514994 Free PMC article.
References
-
- Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1:75-84. - PMC - PubMed
-
- Bijlsma, J. J., and E. A. Groisman. 2003. Making informed decisions: regulatory interactions between two-component systems. Trends Microbiol. 11:359-366. - PubMed
-
- Brennan, M. J., and G. Delogu. 2002. The PE multigene family: a “molecular mantra” for mycobacteria. Trends Microbiol. 10:246-249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

