Identification of a new HLA-A*0201-restricted CD8+ T cell epitope from hepatocellular carcinoma-associated antigen HCA587
- PMID: 15807856
- PMCID: PMC1809362
- DOI: 10.1111/j.1365-2249.2005.02786.x
Identification of a new HLA-A*0201-restricted CD8+ T cell epitope from hepatocellular carcinoma-associated antigen HCA587
Abstract
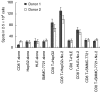
For the development of peptide-based cancer immunotherapies, we aimed to identify specific HLA-A*0201-restricted CTL epitopes in hepatocellular carcinoma (HCC) associated antigen HCA587, which has been identified as a member of the cancer/testis (CT) antigens highly expressed in HCC. We first combined the use of an HLA-A*0201/peptide binding algorithm and T2 binding assays with the induction of specific CD8(+) T cell lines from normal donors by in vitro priming with high-affinity peptides, then IFN-gamma release and cytotoxicity assays were employed to identify the specific HLA-A*0201 CD8(+) T cell epitope using peptide-loaded T2 cells or the HCA587 protein(+) HCC cell line HepG2. In the six candidate synthesized peptides, two peptides showed higher binding ability in T2 binding assays. No. 2 peptide, encompassing amino acid residues FLAKLNNTV (HCA587(317-325)), was able to activate a HCA587-specific CD8(+) T-cell response in human lymphocyte cultures from two normal donors and two HCC patients, and these HCA587-specific CD8(+) T cells recognized peptide-pulsed T2 cells as well as the HCA587 protein(+) HCC cell line HepG2 in IFN-gamma release and cytotoxicity assays. The results indicate that no. 2 peptide is a new HLA-A*0201-restricted CTL epitope capable of inducing HCA587-specific CTLs. Our data suggest that identification of this new HCA587/HLA-A*0201 peptide FLAKLNNTV may facilitate the design of peptide-based immunotherapies for the treatment of HCA587-bearing HCC patients.
Figures






Similar articles
-
Identification of new cytotoxic T-lymphocyte epitopes from cancer testis antigen HCA587.Biochem Biophys Res Commun. 2008 Jul 25;372(2):331-5. doi: 10.1016/j.bbrc.2008.05.049. Epub 2008 May 20. Biochem Biophys Res Commun. 2008. PMID: 18498761
-
Identification of promiscuous HLA-DR-restricted CD4⁺ T-cell epitopes on the cancer-testis antigen HCA587.Cancer Sci. 2011 Aug;102(8):1455-61. doi: 10.1111/j.1349-7006.2011.01986.x. Epub 2011 Jun 27. Cancer Sci. 2011. PMID: 21595801
-
Identification of HLA-A*0201-restricted cytotoxic T lymphocyte epitope from TRAG-3 antigen.Clin Cancer Res. 2003 May;9(5):1850-7. Clin Cancer Res. 2003. PMID: 12738743
-
How frequently are predicted peptides actually recognized by CD8 cells?Cancer Immunol Immunother. 2016 Jul;65(7):847-55. doi: 10.1007/s00262-016-1840-7. Epub 2016 Apr 23. Cancer Immunol Immunother. 2016. PMID: 27108305 Free PMC article. Review.
-
Reverse immunology approach for the identification of CD8 T-cell-defined antigens: advantages and hurdles.Immunol Cell Biol. 2006 Jun;84(3):318-30. doi: 10.1111/j.1440-1711.2006.01447.x. Immunol Cell Biol. 2006. PMID: 16681829 Review.
Cited by
-
MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines.Ther Adv Vaccines. 2014 May;2(3):77-89. doi: 10.1177/2051013614525375. Ther Adv Vaccines. 2014. PMID: 24790732 Free PMC article. Review.
-
Hepatocellular carcinoma-associated antigen 59 of Haemonchus contortus modulates the functions of PBMCs and the differentiation and maturation of monocyte-derived dendritic cells of goats in vitro.Parasit Vectors. 2019 Mar 14;12(1):105. doi: 10.1186/s13071-019-3375-1. Parasit Vectors. 2019. PMID: 30871600 Free PMC article.
-
Induction of HCA587-specific antitumor immunity with HCA587 protein formulated with CpG and ISCOM in mice.PLoS One. 2012;7(10):e47219. doi: 10.1371/journal.pone.0047219. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071764 Free PMC article.
-
MicroRNA-874 Functions as a Tumor Suppressor by Targeting Cancer/Testis Antigen HCA587/MAGE-C2.J Cancer. 2016 Mar 20;7(6):656-63. doi: 10.7150/jca.13674. eCollection 2016. J Cancer. 2016. PMID: 27076846 Free PMC article.
-
Developing effective tumor vaccines: basis, challenges and perspectives.Front Med China. 2007 Feb;1(1):11-9. doi: 10.1007/s11684-007-0003-9. Epub 2007 Feb 1. Front Med China. 2007. PMID: 24557610
References
-
- Schafer DE, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–7. - PubMed
-
- Butterfield LH, Ribas A. Immunotherapy of hepatocellular carcinoma. Expert Opin Opin Biol. 2002;2:123–33. - PubMed
-
- Roth C, Rochlitz C, Kourilsky P. Immune response against tumors. Adv Immunol. 1994;7:281–351. - PubMed
-
- Toes RE, Kast WM, Blom RJ, Bakker SC, Offringa R, Melief CJ. Efficient tumor eradication by adoptively transferred cytotoxic T-cell clones in allogeneic hosts. Int J Cancer. 1996;66:686–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

