N-of-1 trials of quinine efficacy in skeletal muscle cramps of the leg
- PMID: 15808032
- PMCID: PMC1463087
N-of-1 trials of quinine efficacy in skeletal muscle cramps of the leg
Abstract
Background: Skeletal muscle cramps affect over a third of the ambulatory elderly population. Quinine is the established treatment, but there are safety concerns, and evidence for efficacy is conflicting. A recent meta-analysis established a small advantage for quinine, but identified the need for additional studies. N-of-1 trials compare two treatments, in a randomised, double-blind, multiple crossover study on a patient-by-patient basis. They have been used to compare treatments in osteoarthritis and may be suitable for determining the individual efficacy of quinine.
Aim: To establish efficacy and safety of quinine sulphate use for the treatment of leg-muscle cramp.
Design of study: Double-blind, randomised series of n-of-1 controlled trials of quinine versus placebo for muscle cramps.
Setting: New Zealand general practices.
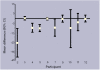
Method: The participants were 13 general practice patients (six males; seven females; median age = 75 years) already prescribed quinine. Following a 2-week washout, each patient received three 4-week treatment blocks of quinine sulphate and matched placebo capsules with an individual, randomised crossover design. The main outcome measures were: patient diaries of cramp occurrence, duration and severity; capsule counts; and blood quinine levels in the final treatment block.
Results: Ten patients completed the trial. Three patients were identified for whom quinine was clearly beneficial (P <0.05), six showed non-significant benefit and one showed no benefit. All patients elected to continue quinine post-study.
Conclusion: Series of n -of-1 studies differentiated patients whom quinine had statistically significant effects; those with trend towards effectiveness; those for whom quinine was probably not effective. Ideally n-of-1 trial should be performed when a patient is commenced on quinine. More cycles in n-of-1 studies of quinine may address issues of statistical power.
Figures
Comment in
-
N-of-1 trials: an opportunity to tailor treatment in individual patients.Br J Gen Pract. 2005 Mar;55(512):171-2. Br J Gen Pract. 2005. PMID: 15808030 Free PMC article. No abstract available.
References
-
- Stewart RB, Moore MT, Marks RG, Hale WE. Nocturnal leg cramps in an ambulatory elderly population: An evaluation of risk factors. Journal of Geriatric Drug Therapy. 1993;7:23–46.
-
- Maule B. Nocturnal cramp: quinine versus folklore. Practitioner. 1990;234:420–421. - PubMed
-
- Daniel H. Simple cure for nocturnal leg cramps. N Engl J Med. 1979;301:216. - PubMed
-
- McGee SR. Muscle cramps. Arch Intern Med. 1990;150:511–518. - PubMed
-
- Mandal AK, Abernathy T, Nelluri SN, Stitzel V. Is quinine effective and safe in leg cramps? J Clin Pharmacol. 1995;35:588–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials