Molecular analyses of disease pathogenesis: application of bovine microarrays
- PMID: 15808306
- PMCID: PMC7112672
- DOI: 10.1016/j.vetimm.2005.02.015
Molecular analyses of disease pathogenesis: application of bovine microarrays
Abstract
The molecular analysis of disease pathogenesis in cattle has been limited by the lack of availability of tools to analyze both host and pathogen responses. These limitations are disappearing with the advent of methodologies such as microarrays that facilitate rapid characterization of global gene expression at the level of individual cells and tissues. The present review focuses on the use of microarray technologies to investigate the functional pathogenomics of infectious disease in cattle. We discuss a number of unique issues that must be addressed when designing both in vitro and in vivo model systems to analyze host responses to a specific pathogen. Furthermore, comparative functional genomic strategies are discussed that can be used to address questions regarding host responses that are either common to a variety of pathogens or unique to individual pathogens. These strategies can also be applied to investigations of cell signaling pathways and the analyses of innate immune responses. Microarray analyses of both host and pathogen responses hold substantial promise for the generation of databases that can be used in the future to address a wide variety of questions. A critical component limiting these comparative analyses will be the quality of the databases and the complete functional annotation of the bovine genome. These limitations are discussed with an indication of future developments that will accelerate the validation of data generated when completing a molecular characterization of disease pathogenesis in cattle.
Figures
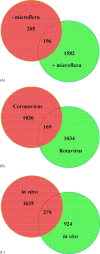
Similar articles
-
The expanding role of microarrays in the investigation of macrophage responses to pathogens.Vet Immunol Immunopathol. 2005 May 15;105(3-4):259-75. doi: 10.1016/j.vetimm.2005.02.001. Vet Immunol Immunopathol. 2005. PMID: 15808305
-
Use of a candidate gene array to delineate gene expression patterns in cattle selected for resistance or susceptibility to intestinal nematodes.Vet Parasitol. 2009 May 26;162(1-2):106-15. doi: 10.1016/j.vetpar.2008.12.017. Epub 2008 Dec 25. Vet Parasitol. 2009. PMID: 19375862
-
Mastitis associated transcriptomic disruptions in cattle.Vet Immunol Immunopathol. 2010 Dec 15;138(4):267-79. doi: 10.1016/j.vetimm.2010.10.005. Epub 2010 Oct 30. Vet Immunol Immunopathol. 2010. PMID: 21040982 Review.
-
Development of a novel equine whole transcript oligonucleotide GeneChip microarray and its use in gene expression profiling of normal articular-epiphyseal cartilage.Equine Vet J. 2009 Sep;41(7):663-70. doi: 10.2746/042516409x412381. Equine Vet J. 2009. PMID: 19927585
-
Genomic and transcriptomic studies in Mycobacterium avium subspecies paratuberculosis.Vet Immunol Immunopathol. 2010 Dec 15;138(4):303-11. doi: 10.1016/j.vetimm.2010.10.008. Epub 2010 Nov 2. Vet Immunol Immunopathol. 2010. PMID: 21047690 Review.
Cited by
-
Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's disease.Infect Immun. 2012 Sep;80(9):3039-48. doi: 10.1128/IAI.00406-12. Epub 2012 Jun 11. Infect Immun. 2012. PMID: 22689821 Free PMC article.
-
Farm animal genomics and informatics: an update.Nucleic Acids Res. 2005 Nov 7;33(19):6308-18. doi: 10.1093/nar/gki931. Print 2005. Nucleic Acids Res. 2005. PMID: 16275782 Free PMC article. Review.
-
Gene expression profiling of bovine bronchial epithelial cells exposed in vitro to bovine herpesvirus 1 and Mannheimia haemolytica.Vet Immunol Immunopathol. 2013 Sep 15;155(3):182-9. doi: 10.1016/j.vetimm.2013.06.012. Epub 2013 Jul 1. Vet Immunol Immunopathol. 2013. PMID: 23890750 Free PMC article.
-
Application of Functional Genomics for Bovine Respiratory Disease Diagnostics.Bioinform Biol Insights. 2015 Oct 22;9(Suppl 2):13-23. doi: 10.4137/BBI.S30525. eCollection 2015. Bioinform Biol Insights. 2015. PMID: 26526746 Free PMC article. Review.
-
Modern approaches to understanding stress and disease susceptibility: A review with special emphasis on respiratory disease.Int J Gen Med. 2009 Jul 30;2:19-32. doi: 10.2147/ijgm.s4843. Int J Gen Med. 2009. PMID: 20360883 Free PMC article.
References
-
- Aich, P., Wilson, H.L., Rawlyk, N., Jalal, S., Kaushik, R.S., Begg, A.A., Potter, A., Babiuk, L.A., Abrahamsen, M.S., Griebel, P., 2005. Microarray analysis of gene expression following preparation of sterile intestinal “loops” in calves. Can. J. Anim. Sci., in press.
-
- Ball C.A., Sherlock G., Parkinson H., Rocca-Sera P., Brooksbank C., Causton H.C., Cavalieri D., Gaasterland T., Hingamp P., Holstege F., Ringwald M., Spellman P., Stoeckert C.J., Jr., Stewart J.E., Taylor R., Brazma A., Quackenbush J. Standards for microarray data. Science. 2002;298(5593):539. - PubMed
-
- Band M.R., Olmstead C., Everts R.E., Liu Z.L., Lewin H.A. A 3800 gene microarray for cattle functional genomics: comparison of gene expression in spleen, placenta, and brain. Anim. Biotechnol. 2002;13(1):163–172. - PubMed
-
- Bao P., Frutos A.G., Greef C., Lahiri J., Muller U., Peterson T.C., Warden L., Xie X. High-sensitivity detection of DNA hybridization on microarrays using resonance light scattering. Anal. Chem. 2002;74(8):1792–1797. - PubMed
-
- Barlow C., Lockhart D.J. DNA arrays and neurobiology—what's new and what's next? Curr. Opin. Neurobiol. 2002;12(5):554–561. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

