Phenylbutyrate up-regulates the adrenoleukodystrophy-related gene as a nonclassical peroxisome proliferator
- PMID: 15809314
- PMCID: PMC2171887
- DOI: 10.1083/jcb.200501036
Phenylbutyrate up-regulates the adrenoleukodystrophy-related gene as a nonclassical peroxisome proliferator
Abstract
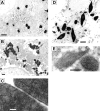
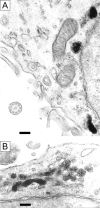
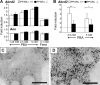
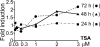
X-linked adrenoleukodystrophy (X-ALD) is a demyelinating disease due to mutations in the ABCD1 (ALD) gene, encoding a peroxisomal ATP-binding cassette transporter (ALDP). Overexpression of adrenoleukodystrophy-related protein, an ALDP homologue encoded by the ABCD2 (adrenoleukodystrophy-related) gene, can compensate for ALDP deficiency. 4-Phenylbutyrate (PBA) has been shown to induce both ABCD2 expression and peroxisome proliferation in human fibroblasts. We show that peroxisome proliferation with unusual shapes and clusters occurred in liver of PBA-treated rodents in a PPARalpha-independent way. PBA activated Abcd2 in cultured glial cells, making PBA a candidate drug for therapy of X-ALD. The Abcd2 induction observed was partially PPARalpha independent in hepatocytes and totally independent in fibroblasts. We demonstrate that a GC box and a CCAAT box of the Abcd2 promoter are the key elements of the PBA-dependent Abcd2 induction, histone deacetylase (HDAC)1 being recruited by the GC box. Thus, PBA is a nonclassical peroxisome proliferator inducing pleiotropic effects, including effects at the peroxisomal level mainly through HDAC inhibition.
Figures





Similar articles
-
Metformin-induced mitochondrial function and ABCD2 up-regulation in X-linked adrenoleukodystrophy involves AMP-activated protein kinase.J Neurochem. 2016 Jul;138(1):86-100. doi: 10.1111/jnc.13562. Epub 2016 Mar 14. J Neurochem. 2016. PMID: 26849413
-
Fibrate induction of the adrenoleukodystrophy-related gene (ABCD2): promoter analysis and role of the peroxisome proliferator-activated receptor PPARalpha.Eur J Biochem. 2001 Jun;268(12):3490-500. doi: 10.1046/j.1432-1327.2001.02249.x. Eur J Biochem. 2001. PMID: 11422379
-
Dehydroepiandrosterone up-regulates the Adrenoleukodystrophy-related gene (ABCD2) independently of PPARalpha in rodents.Biochimie. 2007 Nov;89(11):1312-21. doi: 10.1016/j.biochi.2007.06.013. Epub 2007 Jul 6. Biochimie. 2007. PMID: 17686565
-
Regulation of the adrenoleukodystrophy-related gene (ABCD2): focus on oxysterols and LXR antagonists.Biochem Biophys Res Commun. 2014 Apr 11;446(3):651-5. doi: 10.1016/j.bbrc.2014.01.025. Epub 2014 Jan 27. Biochem Biophys Res Commun. 2014. PMID: 24480443 Review.
-
X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment.Mol Genet Metab. 2007 Mar;90(3):268-76. doi: 10.1016/j.ymgme.2006.10.001. Epub 2006 Nov 7. Mol Genet Metab. 2007. PMID: 17092750 Review.
Cited by
-
Peroxisomal dysfunction in inflammatory childhood white matter disorders: an unexpected contributor to neuropathology.J Child Neurol. 2009 Sep;24(9):1147-57. doi: 10.1177/0883073809338327. Epub 2009 Jul 15. J Child Neurol. 2009. PMID: 19605772 Free PMC article. Review.
-
Inside job: ligand-receptor pharmacology beneath the plasma membrane.Acta Pharmacol Sin. 2013 Jul;34(7):859-69. doi: 10.1038/aps.2013.51. Epub 2013 May 20. Acta Pharmacol Sin. 2013. PMID: 23685953 Free PMC article. Review.
-
The Peroxisomal 3-keto-acyl-CoA thiolase B Gene Expression Is under the Dual Control of PPARα and HNF4α in the Liver.PPAR Res. 2010;2010:352957. doi: 10.1155/2010/352957. Epub 2011 Mar 6. PPAR Res. 2010. PMID: 21437216 Free PMC article.
-
Histone deacetylase inhibitor upregulates peroxisomal fatty acid oxidation and inhibits apoptotic cell death in abcd1-deficient glial cells.PLoS One. 2013 Jul 26;8(7):e70712. doi: 10.1371/journal.pone.0070712. Print 2013. PLoS One. 2013. PMID: 23923017 Free PMC article.
-
A Thyroid Hormone-Based Strategy for Correcting the Biochemical Abnormality in X-Linked Adrenoleukodystrophy.Endocrinology. 2017 May 1;158(5):1328-1338. doi: 10.1210/en.2016-1842. Endocrinology. 2017. PMID: 28200172 Free PMC article.
References
-
- Abe, I., and Y. Fujiki. 1998. cDNA cloning and characterization of a constitutively expressed isoform of the human peroxin Pex11p. Biochem. Biophys. Res. Commun. 252:529–533. - PubMed
-
- Abe, I., K. Okumoto, S. Tamura, and Y. Fujiki. 1998. Clofibrate-inducible, 28-kDa peroxisomal integral membrane protein is encoded by PEX11. FEBS Lett. 431:468–472. - PubMed
-
- Albet, S., C. Causeret, M. Bentejac, J.L. Mandel, P. Aubourg, and M. Bugaut. 1997. Fenofibrate differently alters expression of genes encoding ATP-binding transporter proteins of the peroxisomal membrane. FEBS Lett. 405:394–397. - PubMed
-
- Albet, S., M. Bentejac, S. Savary, C. Gondcaille, A. Netik, J. Berger, C. Szpirer, N. Troffer-Charlier, and M. Bugaut. 2001. Rat adrenoleukodystrophy-related (ALDR) gene: full-length cDNA sequence and new insight in expression. Biochim. Biophys. Acta. 1517:257–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

