IL-1 receptor-associated kinase M is a central regulator of osteoclast differentiation and activation
- PMID: 15809356
- PMCID: PMC2213136
- DOI: 10.1084/jem.20041444
IL-1 receptor-associated kinase M is a central regulator of osteoclast differentiation and activation
Abstract
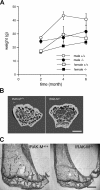
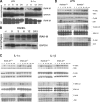
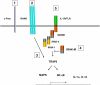
Osteoporosis is a serious problem worldwide; it is characterized by bone fractures in response to relatively mild trauma. Osteoclasts originate from the fusion of macrophages and they play a central role in bone development and remodeling via the resorption of bone. Therefore, osteoclasts are important mediators of bone loss that leads, for example, to osteoporosis. Interleukin (IL)-1 receptor (IL-1R)-associated kinase M (IRAK-M) is only expressed in cells of the myeloid lineage and it inhibits signaling downstream of IL-1R and Toll-like receptors (TLRs). However, it lacks a functional catalytic site and, thus, cannot function as a kinase. IRAK-M associates with, and prevents the dissociation of, IRAK-IRAK-4-TNF receptor-associated factor 6 from the TLR signaling complex, with resultant disruption of downstream signaling. Thus, IRAK-M acts as a dominant negative IRAK. We show here that mice that lack IRAK-M develop severe osteoporosis, which is associated with the accelerated differentiation of osteoclasts, an increase in the half-life of osteoclasts, and their activation. Ligation of IL-1R or TLRs results in hyperactivation of NF-kappaB and mitogen-activated protein kinase signaling pathways, which are essential for osteoclast differentiation. Thus, IRAK-M is a key regulator of the bone loss that is due to osteoclastic resorption of bone.
Figures


Similar articles
-
6-Methylprednisolone down-regulates IRAK-M in human and murine osteoclasts and boosts bone-resorbing activity: a putative mechanism for corticoid-induced osteoporosis.J Leukoc Biol. 2007 Sep;82(3):700-9. doi: 10.1189/jlb.1106673. Epub 2007 Jun 18. J Leukoc Biol. 2007. PMID: 17576820
-
Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction.J Exp Med. 2005 Mar 21;201(6):915-23. doi: 10.1084/jem.20042372. Epub 2005 Mar 14. J Exp Med. 2005. PMID: 15767370 Free PMC article.
-
Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3.Nat Immunol. 2004 Jan;5(1):98-103. doi: 10.1038/ni1014. Epub 2003 Dec 7. Nat Immunol. 2004. PMID: 14661019
-
Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members.Mol Cell. 2003 Feb;11(2):293-302. doi: 10.1016/s1097-2765(03)00053-4. Mol Cell. 2003. PMID: 12620219 Review.
-
IRAK-4 as the central TIR signaling mediator in innate immunity.Trends Immunol. 2002 Oct;23(10):503-6. doi: 10.1016/s1471-4906(02)02298-6. Trends Immunol. 2002. PMID: 12297423 Review.
Cited by
-
Osteoimmunology: interactions of the bone and immune system.Endocr Rev. 2008 Jun;29(4):403-40. doi: 10.1210/er.2007-0038. Epub 2008 May 1. Endocr Rev. 2008. PMID: 18451259 Free PMC article. Review.
-
Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy.J Am Soc Nephrol. 2014 Feb;25(2):292-304. doi: 10.1681/ASN.2013020152. Epub 2013 Dec 5. J Am Soc Nephrol. 2014. PMID: 24309188 Free PMC article.
-
A CD40 variant is associated with systemic bone loss among patients with rheumatoid arthritis.Clin Rheumatol. 2022 Jun;41(6):1851-1858. doi: 10.1007/s10067-021-05998-9. Epub 2022 Feb 2. Clin Rheumatol. 2022. PMID: 35107652
-
Modulating inflammation through the negative regulation of NF-κB signaling.J Leukoc Biol. 2018 Feb 1:10.1002/JLB.3MIR0817-346RRR. doi: 10.1002/JLB.3MIR0817-346RRR. Online ahead of print. J Leukoc Biol. 2018. PMID: 29389019 Free PMC article. Review.
-
Identification of candidate genes in osteoporosis by integrated microarray analysis.Bone Joint Res. 2016 Dec;5(12):594-601. doi: 10.1302/2046-3758.512.BJR-2016-0073.R1. Bone Joint Res. 2016. PMID: 27908864 Free PMC article.
References
-
- Yoshida, H., S.-I. Hayashi, T. Kunisada, M. Ogawa, S. Nishikawa, H. Okamura, T. Sudo, L.D. Shultz, and S.-I. Nishikawa. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345:442–444. - PubMed
-
- Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, et al. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA. 31:3597–3602. - PMC - PubMed
-
- Lacey, D.L., E. Timms, H.L. Tan, M.J. Kelley, C.R. Dunstan, T. Burgess, R. Elliott, A. Colombero, G. Elliott, S. Scully, et al. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 93:165–176. - PubMed
-
- Simonet, W.S., D.L. Lacey, C.R. Dunstan, M. Kelley, M.-S. Chang, R. Lüthy, H.Q. Nguyen, S. Wooden, L. Bennett, T. Boone, et al. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 18:309–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

