Essential role of endothelial Notch1 in angiogenesis
- PMID: 15809373
- PMCID: PMC2633594
- DOI: 10.1161/01.CIR.0000160870.93058.DD
Essential role of endothelial Notch1 in angiogenesis
Abstract
Background: Notch signaling influences binary cell fate decisions in a variety of tissues. The Notch1 receptor is widely expressed during embryogenesis and is essential for embryonic development. Loss of global Notch1 function results in early embryonic lethality, but the cell type responsible for this defect is not known. Here, we identify the endothelium as the primary target tissue affected by Notch1 signaling.
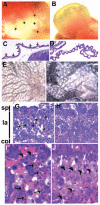
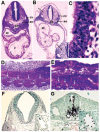
Methods and results: We generated an endothelium-specific deletion of Notch1 using Tie2Cre and conditional Notch1(flox/flox) mice. Mutant embryos lacking endothelial Notch1 died at approximately embryonic day 10.5 with profound vascular defects in placenta, yolk sac, and embryo proper, whereas heterozygous deletion had no effect. In yolk sacs of mutant embryos, endothelial cells formed a primary vascular plexus indicative of intact vasculogenesis but failed to induce the secondary vascular remodeling required to form a mature network of well-organized large and small blood vessels, which demonstrates a defect in angiogenesis. These vascular defects were also evident in the placenta, where blood vessels failed to invade the placental labyrinth, and in the embryo proper, where defective blood vessel maturation led to pericardial and intersomitic hemorrhage. Enhanced activation of caspase-3 was detected in endothelial and neural cells of mutant mice, which resulted in enhanced apoptotic degeneration of somites and the neural tube.
Conclusions: These findings recapitulate the vascular phenotype of global Notch1-/- mutants and indicate an essential cell-autonomous role of Notch1 signaling in the endothelium during vascular development. These results may have important clinical implications with regard to Notch1 signaling in adult angiogenesis.
Figures


References
-
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. - PubMed
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
-
- Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. - PubMed
-
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. - PubMed
-
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

