The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy
- PMID: 15809417
- PMCID: PMC556308
- DOI: 10.1073/pnas.0501634102
The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy
Abstract
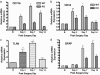
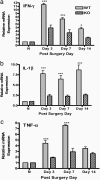
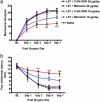
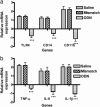
Neuropathic pain remains a prevalent and persistent clinical problem because of our incomplete understanding of its pathogenesis. This study demonstrates for the first time, to our knowledge, a critical role for CNS innate immunity by means of microglial Toll-like receptor 4 (TLR4) in the induction phase of behavioral hypersensitivity in a mouse and rat model of neuropathy. We hypothesized that after L5 nerve transection, CNS neuroimmune activation and subsequent cytokine expression are triggered by the stimulation of microglial membrane-bound TLR4. To test this hypothesis, experiments were undertaken to assess tactile and thermal hypersensitivity in genetically altered (i.e., TLR4 knockout and point-mutant) mice after L5 nerve transection. In a complementary study, TLR4 antisense oligodeoxynucleotide (ODN) was administered intrathecally to L5 spinal nerve injured rats to reduce the expression of spinal TLR4. Both the genetically altered mice and the rats treated with TLR4 antisense ODN displayed significantly attenuated behavioral hypersensitivity and decreased expression of spinal microglial markers and proinflammatory cytokines as compared with their respective control groups. This finding shows that TLR4 contributes to the initiation of CNS neuroimmune activation after L5 nerve transection. Further understanding of this early, specific, innate CNS/microglial response and how it leads to sustained glial/neuronal hypersensitivity may point to new therapies for the prevention and treatment of neuropathic pain syndromes.
Figures


References
-
- DeLeo, J. A. & Yezierski, R. P. (2001) Pain 90, 1–6. - PubMed
-
- DeLeo, J. A., Tanga, F. Y. & Tawfik, V. L. (2004) Neuroscientist 10, 40–52. - PubMed
-
- Vuong, C., Voyich, J. M., Fischer, E. R., Braughton, K. R., Whitney, A. R., DeLeo, F. R. & Otto, M. (2004) Cell. Microbiol. 6, 269–275. - PubMed
-
- Raghavendra, V., Tanga, F. Y. & DeLeo, J. A. (2004) Neuropsychopharmacology 29, 327–334. - PubMed
-
- Sommer, C., Galbraith, J. A., Heckman, H. M. & Myers, R. R. (1993) J. Neuropathol. Exp. Neurol. 52, 223–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

