Dynamics of Z-band based proteins in developing skeletal muscle cells
- PMID: 15810059
- PMCID: PMC1993831
- DOI: 10.1002/cm.20063
Dynamics of Z-band based proteins in developing skeletal muscle cells
Abstract
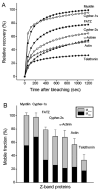
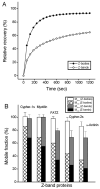
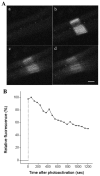
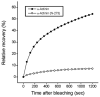
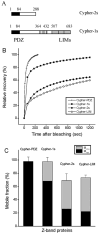
During myofibril formation, Z-bodies, small complexes of alpha-actinin and associated proteins, grow in size, fuse and align to produce Z-bands. To determine if there were changes in protein dynamics during the assembly process, Fluorescence Recovery after Photobleaching was used to measure the exchange of Z-body and Z-band proteins with cytoplasmic pools in cultures of quail myotubes. Myotubes were transfected with plasmids encoding Yellow, Green, or Cyan Fluorescent Protein linked to the Z-band proteins: actin, alpha-actinin, cypher, FATZ, myotilin, and telethonin. Each Z-band protein showed a characteristic recovery rate and mobility. All except telethonin were localized in both Z-bodies and Z-bands. Proteins that were present both early in development in Z-bodies and later in Z-bands had faster exchange rates in Z-bodies. These results suggest that during myofibrillogenesis, molecular interactions develop between the Z-band proteins that decrease their mobility and increase the stability of the Z-bands. A truncated construct of alpha-actinin, which localized in Z-bands in myotubes and exhibited a very low rate of exchange, led to disruption of myofibrils, suggesting the importance of dynamic, intact alpha-actinin molecules for the formation and maintenance of Z-bands. Our experiments reveal the Z-band to be a much more dynamic structure than its appearance in electron micrographs of cross-striated muscle cells might suggest.
Figures




Similar articles
-
Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging.Cell Motil Cytoskeleton. 2008 May;65(5):353-67. doi: 10.1002/cm.20265. Cell Motil Cytoskeleton. 2008. PMID: 18330906
-
Myotilin dynamics in cardiac and skeletal muscle cells.Cytoskeleton (Hoboken). 2011 Dec;68(12):661-70. doi: 10.1002/cm.20542. Epub 2011 Nov 8. Cytoskeleton (Hoboken). 2011. PMID: 22021208 Free PMC article.
-
Targeting of cardiac muscle titin fragments to the Z-bands and dense bodies of living muscle and non-muscle cells.Cell Motil Cytoskeleton. 2000 Jan;45(1):67-82. doi: 10.1002/(SICI)1097-0169(200001)45:1<67::AID-CM7>3.0.CO;2-T. Cell Motil Cytoskeleton. 2000. PMID: 10618168
-
Assembly and Maintenance of Myofibrils in Striated Muscle.Handb Exp Pharmacol. 2017;235:39-75. doi: 10.1007/164_2016_53. Handb Exp Pharmacol. 2017. PMID: 27832381 Review.
-
Myofibrillogenesis in skeletal muscle cells.Clin Orthop Relat Res. 2002 Oct;(403 Suppl):S153-62. doi: 10.1097/00003086-200210001-00018. Clin Orthop Relat Res. 2002. PMID: 12394464 Review.
Cited by
-
Exploring cardiac form and function: A length-scale computational biology approach.Wiley Interdiscip Rev Syst Biol Med. 2020 Mar;12(2):e1470. doi: 10.1002/wsbm.1470. Epub 2019 Dec 2. Wiley Interdiscip Rev Syst Biol Med. 2020. PMID: 31793215 Free PMC article. Review.
-
Different localizations and cellular behaviors of leiomodin and tropomodulin in mature cardiomyocyte sarcomeres.Mol Biol Cell. 2010 Oct 1;21(19):3352-61. doi: 10.1091/mbc.E10-02-0109. Epub 2010 Aug 4. Mol Biol Cell. 2010. PMID: 20685966 Free PMC article.
-
A Supramolecular Polymerization Approach to the Growth of the Myofibril.Front Chem. 2019 Jul 16;7:487. doi: 10.3389/fchem.2019.00487. eCollection 2019. Front Chem. 2019. PMID: 31380341 Free PMC article.
-
Inhibition of DNAJ-HSP70 interaction improves strength in muscular dystrophy.J Clin Invest. 2020 Aug 3;130(8):4470-4485. doi: 10.1172/JCI136167. J Clin Invest. 2020. PMID: 32427588 Free PMC article.
-
Differential effects of Latrunculin-A on myofibrils in cultures of skeletal muscle cells: insights into mechanisms of myofibrillogenesis.Cell Motil Cytoskeleton. 2005 Sep;62(1):35-47. doi: 10.1002/cm.20083. Cell Motil Cytoskeleton. 2005. PMID: 16080205 Free PMC article.
References
-
- Ayoob JC, Turnacioglu KK, Mittal B, Sanger JM, Sanger JW. Targeting of cardiac muscle titin fragments to the Z-bands and dense bodies of living muscle and non-muscle cells. Cell Motil Cyotskel. 2000;45:67–82. - PubMed
-
- Ayoob JC, Shaner NC, Sanger JW, Sanger JM. Expression of green or red fluorescent protein (GFP or DsRed) linked proteins in nonmuscle and muscle cells. Mol Biotechnology. 2001;17:65–71. - PubMed
-
- Bandman E. Myosin isoenzyme transitions in muscle development, maturation and disease. Int Rev Cytol. 1985;97:97–131. - PubMed
-
- Bastiaens PIH, Pepperkok R. Observing proteins in their natural habitat: the living cell. Trends Biochem Sci. 2000;25:631–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

