Identification of the immunogenic domains in HBsAg preS1 region using overlapping preS1 fragment fusion proteins
- PMID: 15810073
- PMCID: PMC4305776
- DOI: 10.3748/wjg.v11.i14.2088
Identification of the immunogenic domains in HBsAg preS1 region using overlapping preS1 fragment fusion proteins
Abstract
Aim: The incorporation of hepatitis B virus (HBV) preS1 region into epitope-based vaccines against HBV has been accepted widely, but the incorporate site and size of preS1 sequence is controversial. Therefore our purpose was to further investigate its immunogenic domains for the epitope-based hepatitis B vaccine design.
Methods: Eight GST fusion proteins containing overlapping preS1 fragments in preS1 (21-119) region were expressed in E.coli. Using these purified fusion proteins, the immunogenic domains in preS1 region were identified in detail in mice and humans by Western blot analysis and ELISA.
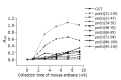
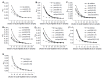
Results: The results in mice showed that the immu-nogenic domains mainly existed in preS1 (21-59) and preS1 (95-109). Similarly, these fragments had strong immunogenicity in humans; whereas the other parts except for preS1 (60-70) also had some immunogenicity. More importantly, a major immunogenic domain, preS1 (34-59), which has much stronger immunogenicity, was identified. Additionally, the antibodies against some preS1 fragments, especially preS1 (34-59), were speculated to be virus-neutralizing.
Conclusion: Eight GST fusion proteins containing overlapping preS1 fragments were prepared successfully. They were used for the study on the immunogenic dom-ains in preS1 (21-119) region. The preS1 (34-59) fragments were the major immunogenic domains in the preS1 region, and the antibodies against these fragments were speculated to be virus-neutralizing. Therefore, the incorporation of preS1 (34-59) fragments into epitope-based HBV vaccines may be efficient for enhancement of immune response. Additionally, the results also imply that there are more complex immune responses to preS1 region and more abundant immunogenic domains in humans.
Figures



Similar articles
-
N-terminal myristoylation-dependent masking of neutralizing epitopes in the preS1 attachment site of hepatitis B virus.J Hepatol. 2011 Jul;55(1):29-37. doi: 10.1016/j.jhep.2010.10.019. Epub 2010 Nov 28. J Hepatol. 2011. PMID: 21145866
-
Expression and immunoactivity of chimeric particulate antigens of receptor binding site-core antigen of hepatitis B virus.World J Gastroenterol. 2005 Jan 28;11(4):492-7. doi: 10.3748/wjg.v11.i4.492. World J Gastroenterol. 2005. PMID: 15641132 Free PMC article.
-
A monoclonal antibody specific to the non-epitope region of hepatitis B virus preS1 contributes to more effective HBV detection.Clin Biochem. 2013 Aug;46(12):1105-1110. doi: 10.1016/j.clinbiochem.2013.04.011. Epub 2013 Apr 19. Clin Biochem. 2013. PMID: 23608352
-
Applications of human hepatitis B virus preS domain in bio- and nanotechnology.World J Gastroenterol. 2015 Jun 28;21(24):7400-11. doi: 10.3748/wjg.v21.i24.7400. World J Gastroenterol. 2015. PMID: 26139986 Free PMC article. Review.
-
Interactions of hepatitis B virus with hepatocytes: mechanism and clinical relevance.Hepatogastroenterology. 1991 Feb;38(1):10-3. Hepatogastroenterology. 1991. PMID: 1709133 Review.
Cited by
-
Occult HBV infection: a faceless enemy in liver cancer development.Viruses. 2014 Apr 8;6(4):1590-611. doi: 10.3390/v6041590. Viruses. 2014. PMID: 24717680 Free PMC article. Review.
-
Pre-structured motifs in the natively unstructured preS1 surface antigen of hepatitis B virus.Protein Sci. 2007 Oct;16(10):2108-17. doi: 10.1110/ps.072983507. Epub 2007 Aug 31. Protein Sci. 2007. PMID: 17766372 Free PMC article.
-
PreS1 Mutations Alter the Large HBsAg Antigenicity of a Hepatitis B Virus Strain Isolated in Bangladesh.Int J Mol Sci. 2020 Jan 15;21(2):546. doi: 10.3390/ijms21020546. Int J Mol Sci. 2020. PMID: 31952213 Free PMC article.
-
Vaccines targeting preS1 domain overcome immune tolerance in hepatitis B virus carrier mice.Hepatology. 2017 Oct;66(4):1067-1082. doi: 10.1002/hep.29239. Epub 2017 Aug 26. Hepatology. 2017. PMID: 28445927 Free PMC article. Clinical Trial.
-
In silico functional and structural characterization of hepatitis B virus PreS/S-gene in Iranian patients infected with chronic hepatitis B virus genotype D.Heliyon. 2020 Jul 15;6(7):e04332. doi: 10.1016/j.heliyon.2020.e04332. eCollection 2020 Jul. Heliyon. 2020. PMID: 32695898 Free PMC article.
References
-
- Ganem D, Varmus HE. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. - PubMed
-
- Beasley RP, Hwang LY. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 1984;4:113–121. - PubMed
-
- Malik AH, Lee WM. Chronic hepatitis B virus infection: treatment strategies for the next millennium. Ann Intern Med. 2000;132:723–731. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

