Effect of hepatitis C virus nonstructural protein NS3 on proliferation and MAPK phosphorylation of normal hepatocyte line
- PMID: 15810084
- PMCID: PMC4305787
- DOI: 10.3748/wjg.v11.i14.2157
Effect of hepatitis C virus nonstructural protein NS3 on proliferation and MAPK phosphorylation of normal hepatocyte line
Abstract
Aim: To study the effect of hepatitis C virus nonstructural region 3 (HCV NS3) protein on proliferation and transformation of normal human liver cell line.
Methods: QSG7701 cells were transfected with pRcHCNS3-5', pRcHCNS3-3' and pRcCMV using lipofectamine transfecting technique and selected with G418 method. Expression of HCV NS3 protein was determined by immunohistochemistry. Biologic characteristics of transfected cells were evaluated by population doubling time and soft agar assays. Activation of MAPK was analyzed using Western blot with phosphospecific monoclonal antibody against dually phosphorylated MAPK.
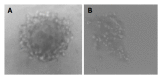
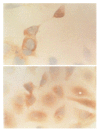
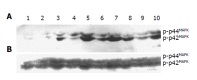
Results: QSG7701 cells transfected with pRcHCNS3-5' showed strong intracellular expression of HCVNS3 protein, and the positive signal was localized in cytoplasm. The expressing strength of HCVNS3 protein in pRcHCNS3-3'-transfected cells was weaker than that in pRcHCNS3-5'-transfected cells. The population doubling time in the transfected cells with pRcHCNS3-5' (12 h) was much shorter than those with pRcHCNS3-3', pRcCMV and normal cells (24, 26, 28 h, respectively) (P<0.01). The transfected cells with pRcHCNS3-5' showed much more anchorage independent colonies than that in those with pRcHCNS3-3' and pRcCMV (P<0.01). The cloning efficiencies of transfected cells with pRcHCNS3-5', pRcHCNS3-3', pRcCMV and controls were 33%, 1.33%, 1.46%, 1.11% respectively. The level of phosphorylated MAPK in the cells with pRcHCNS3-5' was much higher than that in those with pRcHCNS3-3'and pRcCMV and normal cells (P<0.01).
Conclusion: The results suggest that (1) QSG7701 cells are a better human liver cell line for investigating the pathogenesis of HCV NS3 protein. (2) 5' region of the HCV genome segment encoding HCV NS3 is involved in cell growth and cell phenotype. (3) HCV NS3 N-terminal peptide may up-regulate the activation of MAPK, but not affect the expression of MAPK.
Figures



Similar articles
-
Hepatocyte transformation and tumor development induced by hepatitis C virus NS3 c-terminal deleted protein.World J Gastroenterol. 2003 Mar;9(3):474-8. doi: 10.3748/wjg.v9.i3.474. World J Gastroenterol. 2003. PMID: 12632500 Free PMC article.
-
[Hepatocyte transformation and tumor development induced by hepatitis C virus NS3 N-terminal protein].Zhonghua Bing Li Xue Za Zhi. 2003 Jun;32(3):255-9. Zhonghua Bing Li Xue Za Zhi. 2003. PMID: 12882694 Chinese.
-
[Effect of hepatitis C virus nonstructural protein NS3 on telomerase activity].Zhonghua Bing Li Xue Za Zhi. 2001 Dec;30(6):443-7. Zhonghua Bing Li Xue Za Zhi. 2001. PMID: 11866988 Chinese.
-
[Mechanism of hepatocyte transformation by HCV NS3 using two-dimensional electrophoresis and mass spectrometry].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007 Jun;32(3):387-95. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 17611312 Chinese.
-
[Cross-talk between ERK and NF-kappaB signal transduction pathways in the hepatocytes expressing hepatitis C virus nonstructural protein 3].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007 Apr;32(2):259-63. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 17478933 Chinese.
Cited by
-
Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma.World J Gastroenterol. 2020 Oct 14;26(38):5759-5783. doi: 10.3748/wjg.v26.i38.5759. World J Gastroenterol. 2020. PMID: 33132633 Free PMC article. Review.
-
Molecular basis of hepatocellular carcinoma induced by hepatitis C virus infection.World J Hepatol. 2017 Dec 28;9(36):1305-1314. doi: 10.4254/wjh.v9.i36.1305. World J Hepatol. 2017. PMID: 29359013 Free PMC article. Review.
-
Hepatocellular carcinoma after direct-acting antiviral drug treatment in patients with hepatitis C virus.JGH Open. 2018 Nov 9;3(1):52-60. doi: 10.1002/jgh3.12105. eCollection 2019 Feb. JGH Open. 2018. PMID: 30834341 Free PMC article.
-
Hepcidin and the iron enigma in HCV infection.Virulence. 2014 May 15;5(4):465-76. doi: 10.4161/viru.28508. Epub 2014 Mar 13. Virulence. 2014. PMID: 24626108 Free PMC article. Review.
-
Underlying pathophysiology of HCV infection in HIV-positive drug users.J Addict Dis. 2008;27(2):75-82. doi: 10.1300/J069v27n02_09. J Addict Dis. 2008. PMID: 18681194 Free PMC article. Review.
References
-
- Ishido S, Muramatsu S, Fujita T, Iwanaga Y, Tong WY, Katayama Y, Itoh M, Hotta H. Wild-type, but not mutant-type, p53 enhances nuclear accumulation of the NS3 protein of hepatitis C virus. Biochem Biophys Res Commun. 1997;230:431–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

